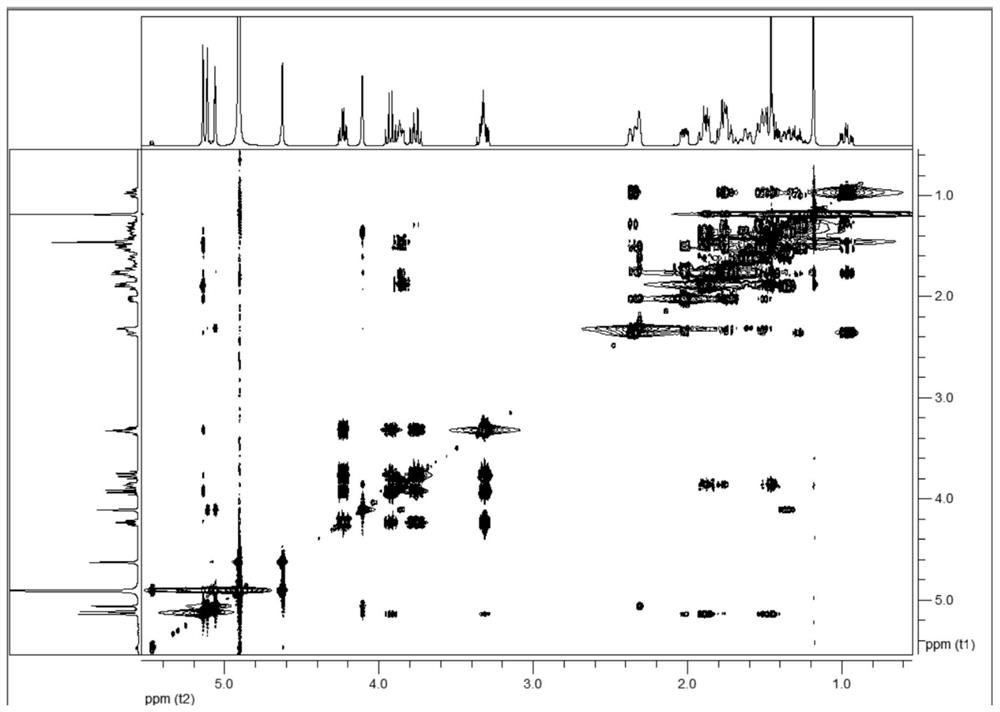
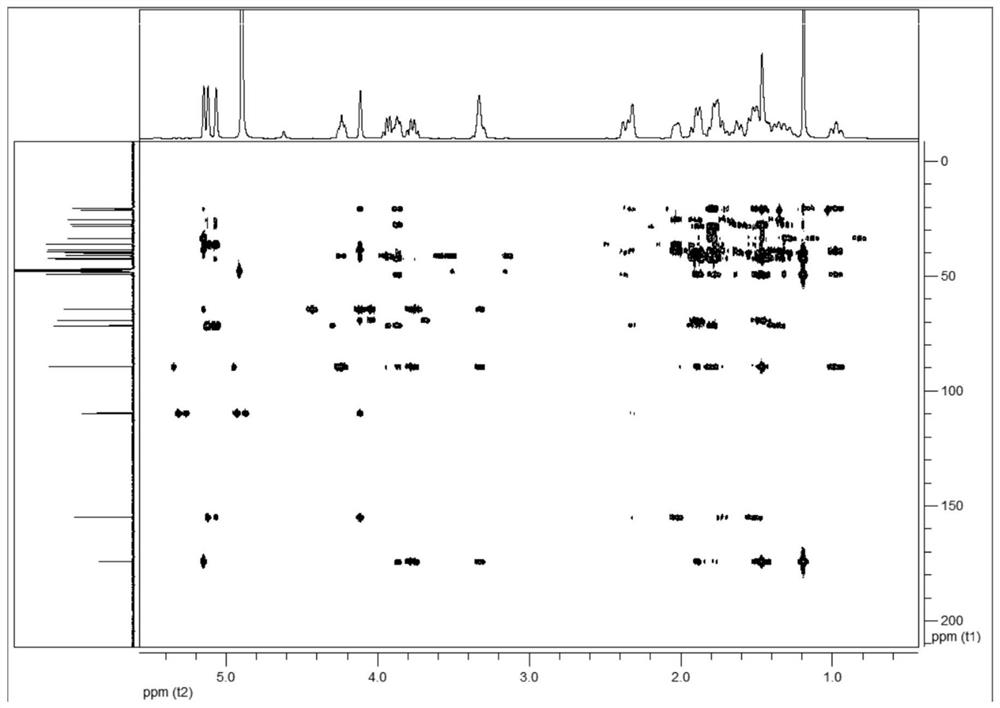
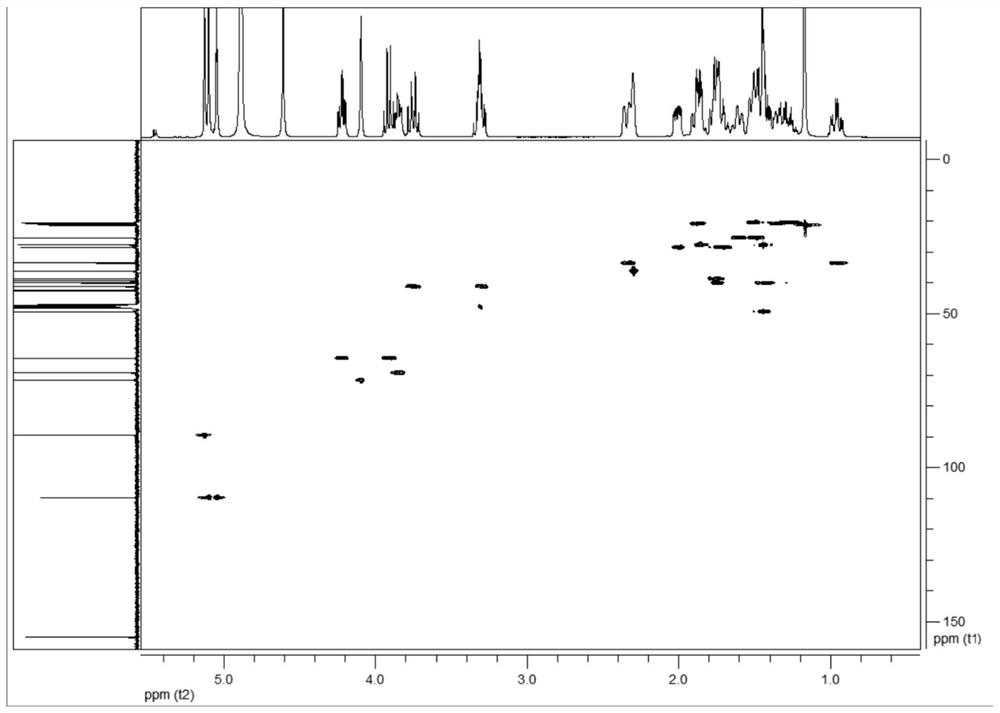
C20-diterpenoid alkaloid compound and preparation method and application thereof
A technology for diterpene alkaloids and compounds, which is applied in the field of altigen-type C20-diterpenoid alkaloids, and can solve unseen problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0115] 1. Preparation of compounds
[0116] (1) Take 5 kg of Delphinium delphinium, immerse and extract with 15 and 10 times of ethanol with a volume concentration of 80% respectively, combine the extracts, recover ethanol and concentrate under reduced pressure until there is no alcohol smell, to obtain 746 g of total extract;
[0117] (2) Dissolving the total extract in water, then, extracting with sherwood oil and ethyl acetate successively, getting the ethyl acetate extract, concentrating under reduced pressure to remove the solvent to obtain 72g of the ethyl acetate extract;
[0118] (3) Mix the ethyl acetate extract into blank silica gel, pass through a silica gel column, and sequentially use dichloromethane-methanol (100:1→2:1) gradient elution; collect dichloromethane-methanol (10:1 ) eluate through a Sephadex LH-20 Sephadex column, eluting with methanol with a volume concentration of 80% as a solvent, collecting the eluate, and concentrating to obtain colorless needle-...
example 2
[0130] Example 2: (tablet)
[0131] Take 2000 mg of the compound obtained by the method described in Example 1 above, mix it with HPMC, lactose and starch in a ratio of 1:9:9:12, granulate with 95% ethanol at 20 mesh, dry below 50°C for 1 hour, 16 mesh Sieve the granules, then add 1% magnesium stearate, mix evenly, and compress into tablets to make tablets with a specification of 100 mg / tablet for oral use.
example 3
[0132] Example 3: (dropping pills)
[0133] Mix 2000 mg of the compound obtained by the method described in Example 1 above with PEG4000 and PEG6000 at a ratio of 1:20:50, melt, drop into the coolant methyl silicone oil to obtain drop pills (50 mg / granule).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com