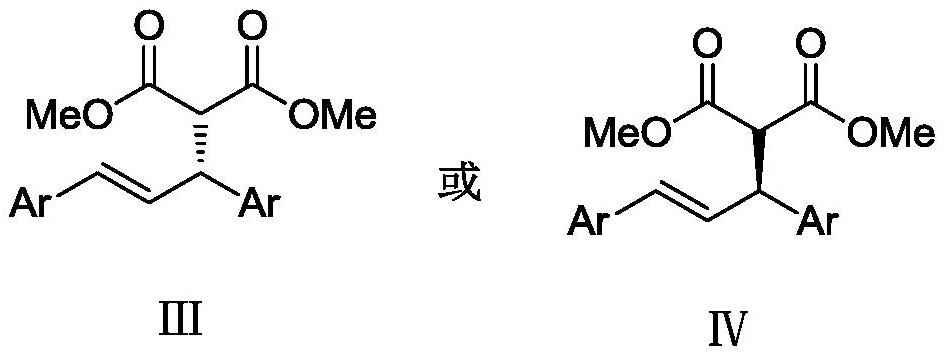
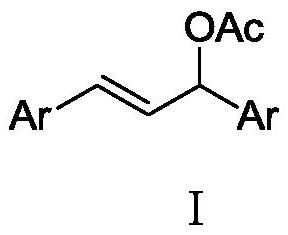
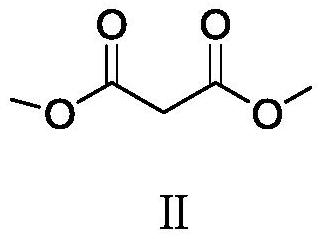
A kind of chiral (e)-2-(1,3-diarylallyl) dimethyl malonate compound and preparation method thereof
A technology of aryl allyl acetate and dimethyl malonate, which is applied in the field of organic synthesis and achieves the effects of high stereoselectivity, good reactivity and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: (n-allyl) PdCl 2 The complexation with L-1-1 is used as a catalyst to catalyze the reaction to generate (E)-2-(1,3-diphenylallyl) dimethyl malonate substituted product III-1.
[0042] Add metal precursor (η-allyl)PdCl to the reaction flask 2 (0.005 mmol, 5 mol %) and chiral ligand L-1-1 (0.011 mmol, 5.5 mol %), 1.0 mL of anhydrous toluene was added under nitrogen protection, and the mixture was stirred at room temperature for 1 hour. Then (E)-1,3-diphenylallyl acetate I-1 (0.5 mmol, 1.0 equiv), dimethyl malonate compound II-1 (1.0 mmol, 3 equiv) and KOAc ( 0.36 mmol, 1.2 equiv) was dissolved in 2.0 mL of anhydrous toluene, then the solution was added to the above stirred catalyst solution under nitrogen protection, and the reaction was stirred at 25° C. for 12 h. After the reaction was completed, it was concentrated under reduced pressure until almost solvent-free, separated by silica gel column chromatography, concentrated under reduced pressure, and dr...
Embodiment 2
[0046] Example 2: L-1-2 reacts as a ligand to generate product III-1
[0047] The ligand L-1-1 in Example 1 was replaced with the ligand L-1-2, and the rest was the same as that in Example 1, and the reaction was carried out to obtain compound III-1, 65% yield, 8.9% ee.
[0048] The structural formula of L-1-2 is as follows:
[0049]
Embodiment 3
[0050] Example 3: L-1-3 reacts as a ligand to generate product III-1
[0051] The ligand L-1-1 in Example 1 was replaced with the ligand L-1-3, and the rest was the same as that in Example 1, and the reaction was carried out to obtain compound III-1, 77% yield, 82% ee.
[0052] The structural formula of L-1-3 is as follows:
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com