Fluorene oxime ester compound, preparation method thereof and photosensitive resin composition
A technology of ester compound and fluorene oxime, applied in the fields of oxime preparation, optics, organic chemistry, etc., can solve the problems of insufficient sensitivity and poor performance of photocurable compositions, achieve good pattern effect, high sensitivity, and solve the problem of sensitivity insufficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
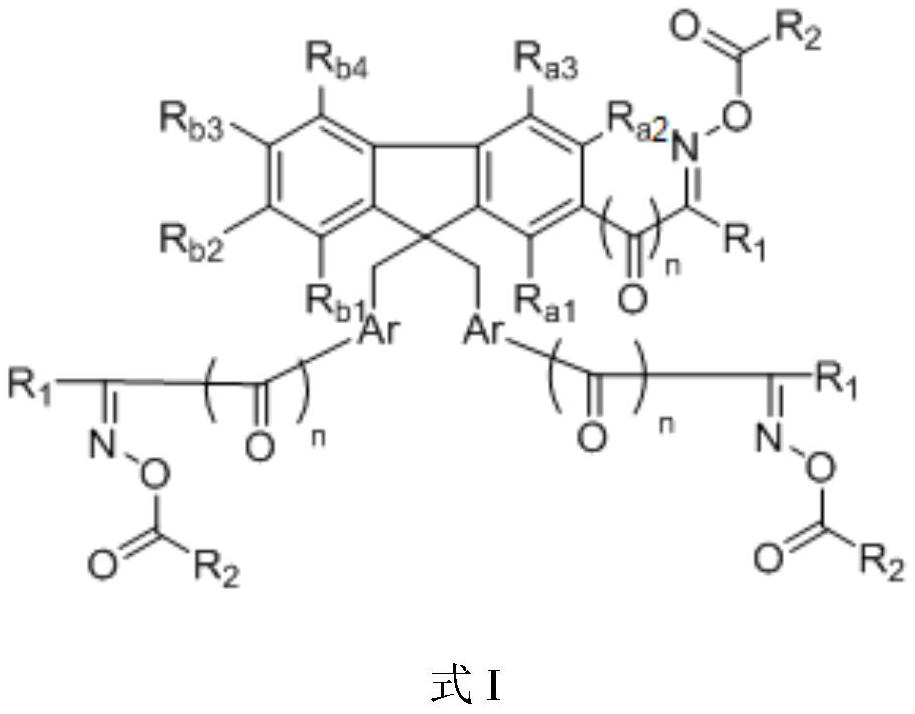
[0032] According to another aspect of the present invention, a kind of preparation method of above-mentioned fluorene oxime ester compound is provided, it comprises the following steps:
[0033] Step S1, performing a Friedel-Crafts reaction on compound a and an acid halide compound under the action of a catalyst to obtain intermediate a; wherein compound a is Intermediate a is R a1 , R a2 , R a3 , R b1 , R b2 , R b3 , R b4 , Ar have the same definition as above; R 1 ' is the residue formed after removing the acid halide group from the acid halide compound;
[0034] In step S2, intermediate a is subjected to an oximation reaction to obtain intermediate b, and intermediate b is where R a1 , R a2 , R a3 , R b1 , R b2 , R b3 , R b4 , Ar, n, R 1 has the same definition as above;
[0035] In step S3, the intermediate b is subjected to an esterification reaction with an acylating agent or an acid anhydride to obtain a fluorene oxime ester compound.
[0036] The ...
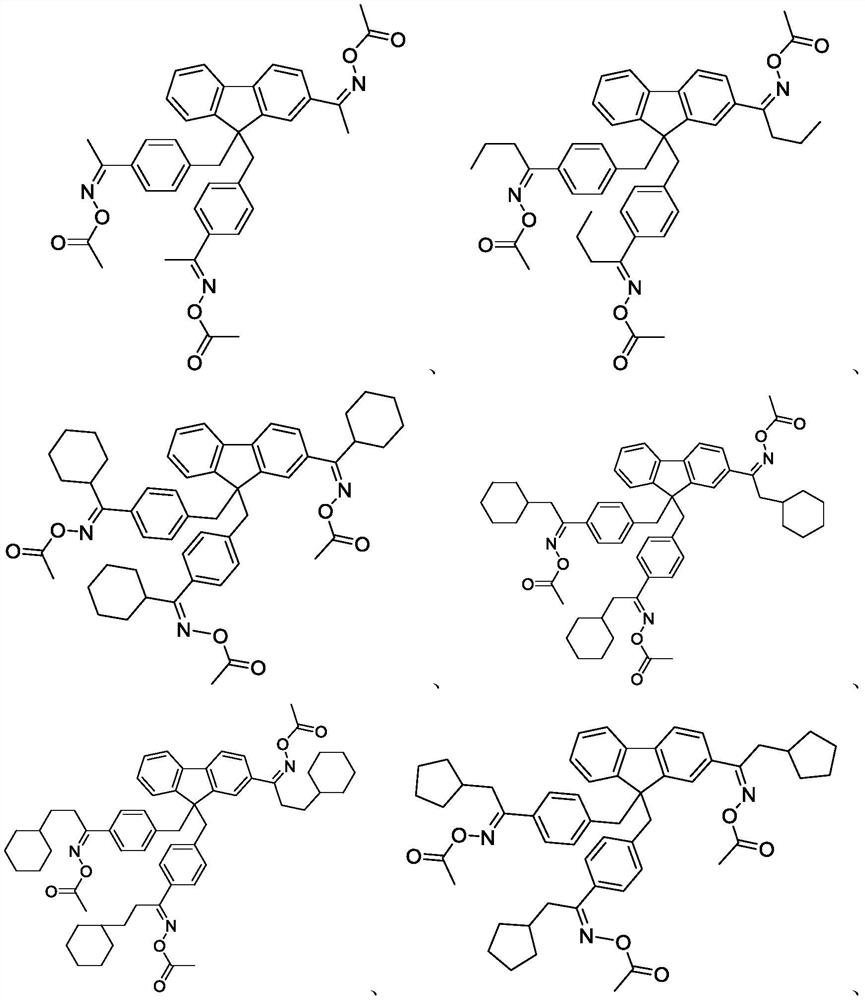
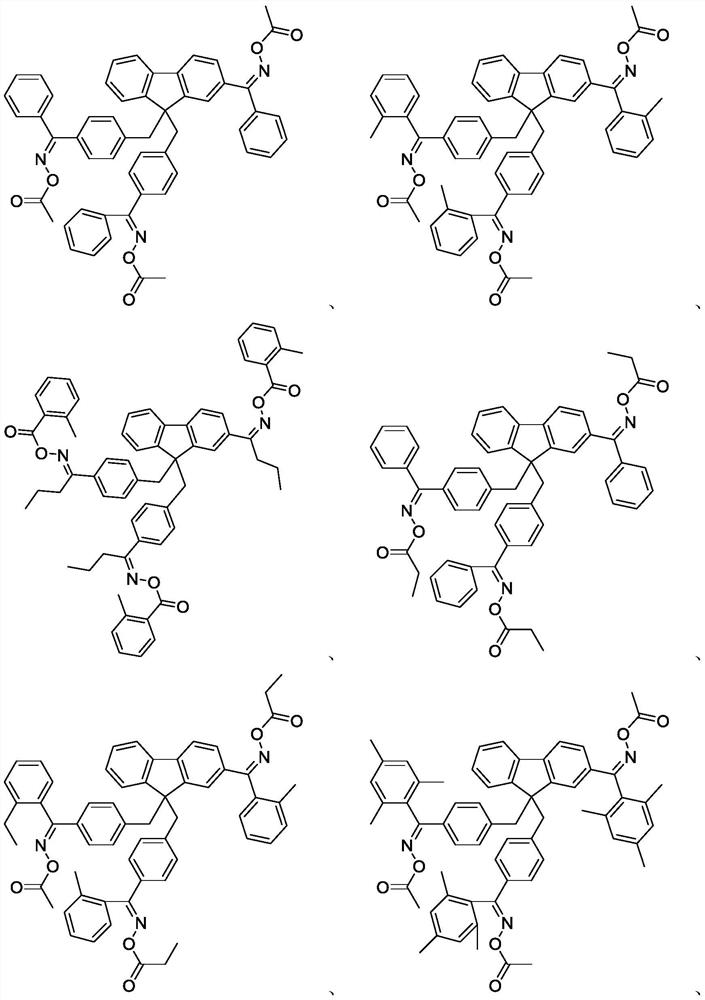
Embodiment 1
[0058] Preparation of Compound A-1
[0059]
[0060] Step (1) Preparation of Intermediate A-1-1
[0061]
[0062] Under the protection of nitrogen, add raw material A-1-0 (103.9g, 0.30mol), aluminum chloride (252.0g, 1.89mol) and 800mL dichloromethane into a 2L reaction flask, stir in an ice bath, and reduce the temperature of the reaction solution to 5-10°C; butyryl chloride (95.9g, 0.90mol) was dissolved in 200mL of dichloromethane, slowly added dropwise to the above reaction solution, the temperature was controlled not to exceed 10°C, after the drop was complete, the reaction was continued for 3h. The reaction solution was slowly added to 1 kg of ice water, fully stirred for 30 minutes, allowed to stand for stratification, and the lower organic phase was separated. The organic phase was washed with water until neutral, and concentrated to obtain a residue. Added 320 g of methanol, stirred to precipitate a solid, and continued to cool down to 5 Continue to stir and cr...
Embodiment 2
[0073] The preparation of embodiment 2 compound A-2
[0074]
[0075] Step (1) Preparation of Intermediate A-2-1
[0076]
[0077] Under nitrogen protection, add raw material A-2-0 (103.9g, 0.30mol), aluminum chloride (336.0g, 2.52mol) and 1000mL dichloromethane into the 2L reaction flask, stir in an ice bath, and reduce the temperature of the reaction solution to 5-10°C; butyryl chloride (127.9g, 1.20mol) was dissolved in 200mL of dichloromethane, slowly added dropwise to the above reaction solution, the temperature was controlled not to exceed 10°C, after the drop was complete, the reaction was continued for 3h. Slowly add the reaction solution into 1 kg of ice water, stir thoroughly for 30 min, let stand to separate layers, separate the lower organic phase, wash the organic phase with water until neutral, concentrate to obtain a residue, add 400 mL of methanol, stir to precipitate a solid, and continue to cool down to 5 Continue to stir and crystallize at -10°C for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com