Preparation method of amorphous boron-doped graphite phase carbon nitride quantum dot, quantum dot prepared by same and application of quantum dot
A graphite-phase carbon nitride and amorphous technology, applied in the field of fluorescent materials, can solve the problems of dependent behavior, self-quenching excitation wavelength, low fluorescence quantum yield, etc., and achieve the promotion of exciton formation, high selectivity and sensitivity , Optimize the effect of optical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
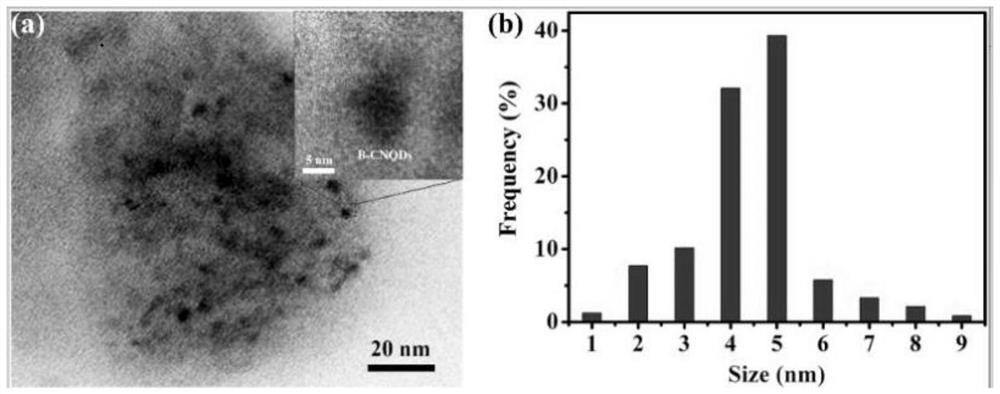
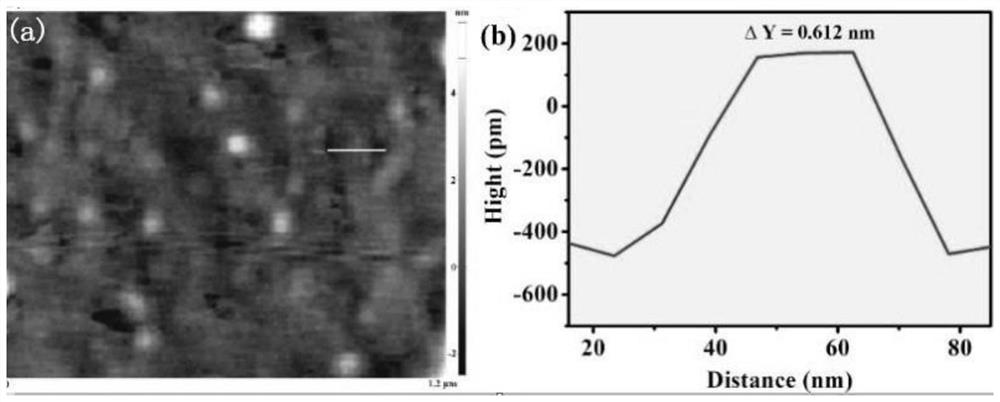
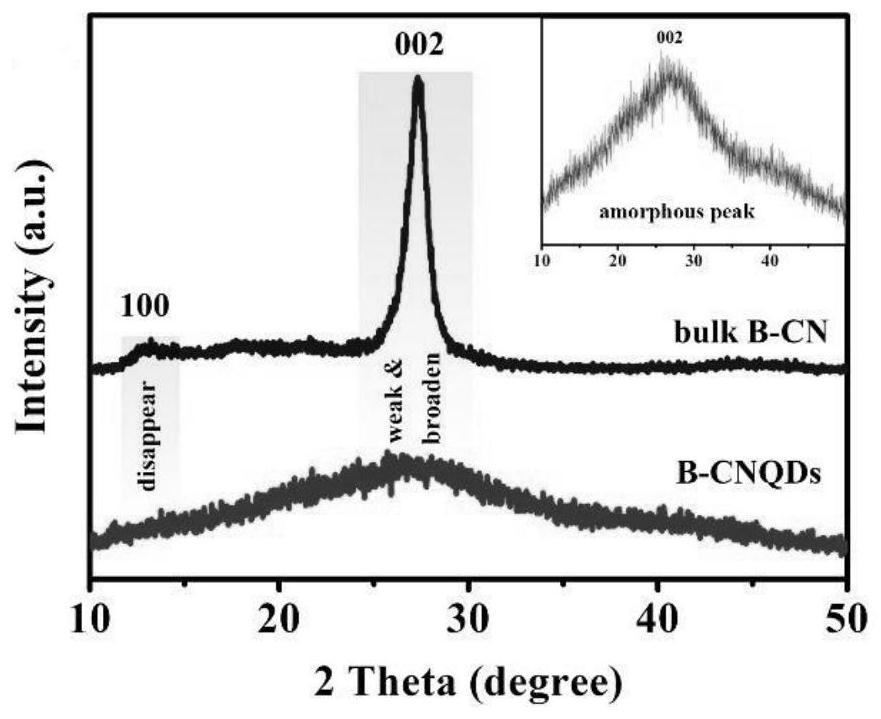
[0038] A preparation method of amorphous boron-doped graphite phase carbon nitride quantum dots (B-CNQDs), comprising the following steps:
[0039] (1) Using citric acid as a carbon source, boric acid as a boron source, and urea as a nitrogen source, add an appropriate amount of deionized water and grind evenly to obtain a mixture;
[0040] (2) Put the mixture into an autoclave and heat up to 180°C for 2 hours;
[0041] (3) naturally cool to normal temperature to obtain a reaction solution, and mix the reaction solution with 4 times the volume of deionized water for ultrasonic treatment;
[0042] (4) The solution obtained in step (3) is collected by membrane filtration to collect the supernatant;
[0043] (5) Dilute the supernatant to obtain the amorphous B-CNQDs suspension.
[0044] Wherein, the mol ratio of citric acid, boric acid, and urea described in step (1) is 1:6:6.
[0045] Wherein, the heating rate described in step (2) is 5° C. / min.
[0046] Wherein, the time of...
Embodiment 2
[0050] A preparation method of amorphous boron-doped graphite phase carbon nitride quantum dots (B-CNQDs), comprising the following steps:
[0051] (1) Using citric acid as a carbon source, boric acid as a boron source, and urea as a nitrogen source, add an appropriate amount of deionized water and grind evenly to obtain a mixture;
[0052] (2) Put the mixture into an autoclave, heat up to 180°C and react for 1.5h;
[0053] (3) naturally cool to normal temperature to obtain a reaction solution, and mix the reaction solution with 4 times the volume of deionized water for ultrasonic treatment;
[0054] (4) The solution obtained in step (3) is collected by membrane filtration to collect the supernatant;
[0055] (5) Dilute the supernatant to obtain the amorphous B-CNQDs suspension.
[0056] Wherein, the mol ratio of citric acid, boric acid, and urea described in step (1) is 1:5:5.
[0057] Wherein, the heating rate in step (2) is 4°C / min.
[0058] Wherein, the time of ultraso...
Embodiment 3
[0062] A preparation method of amorphous boron-doped graphite phase carbon nitride quantum dots (B-CNQDs), comprising the following steps:
[0063] (1) Using citric acid as a carbon source, boric acid as a boron source, and urea as a nitrogen source, add an appropriate amount of deionized water and grind evenly to obtain a mixture;
[0064] (2) Put the mixture into an autoclave, heat up to 180°C and react for 2.5h;
[0065] (3) naturally cool to normal temperature to obtain a reaction solution, and mix the reaction solution with 4 times the volume of deionized water for ultrasonic treatment;
[0066] (4) The solution obtained in step (3) is collected by membrane filtration to collect the supernatant;
[0067] (5) Dilute the supernatant to obtain the amorphous B-CNQDs suspension.
[0068] Wherein, the mol ratio of citric acid, boric acid, and urea described in step (1) is 1:7:7.
[0069] Wherein, the heating rate in step (2) is 6° C. / min.
[0070] Wherein, the time of ultra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com