Cycloolefin copolymer with polar group and preparation method thereof
A cyclic olefin copolymer and polar group technology, applied in the field of C08F, can solve the problems of lack of physical properties of cyclic olefin copolymer, loss of performance advantages of cyclic olefin copolymer, insufficient comonomer insertion rate, etc., to avoid crystallinity. Effects of segment generation, compatibility promotion, and enhancement of optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]Embodiment 1 provides the preparation method of cycloolefin copolymer and the prepared copolymer thereof, including: adding solvent toluene, and a certain amount of norbornene monomer (b) and 5-hydroxy- 2-norbornene monomer (c), the concentrations of monomers (b) and (c) were 2.0 mol / L and 0.5 mol / L, respectively, and the total volume was kept at 200 mL. Start stirring, set the rotation speed at 500r / min, then feed ethylene monomer (a), raise the pressure to 0.5MPa, and equilibrate at 70°C for 30 minutes. Then, 2 mL of catalyst containing 5 μmol (Ar 1 and Ar 2 C respectively 6 h 5 , 2-{(2’,6’-(CH 3 O) 2 (C 6 h 3 )}C 6 h 4 , R 10 , R 11 Respectively CH 3 , H) dichloromethane solution, start the polymerization reaction, keep the system temperature and pressure constant during the polymerization process. After the reaction was carried out for 1 hour, the stirring was stopped, the temperature of the reactor was lowered to room temperature, the pressure was relea...
Embodiment 15
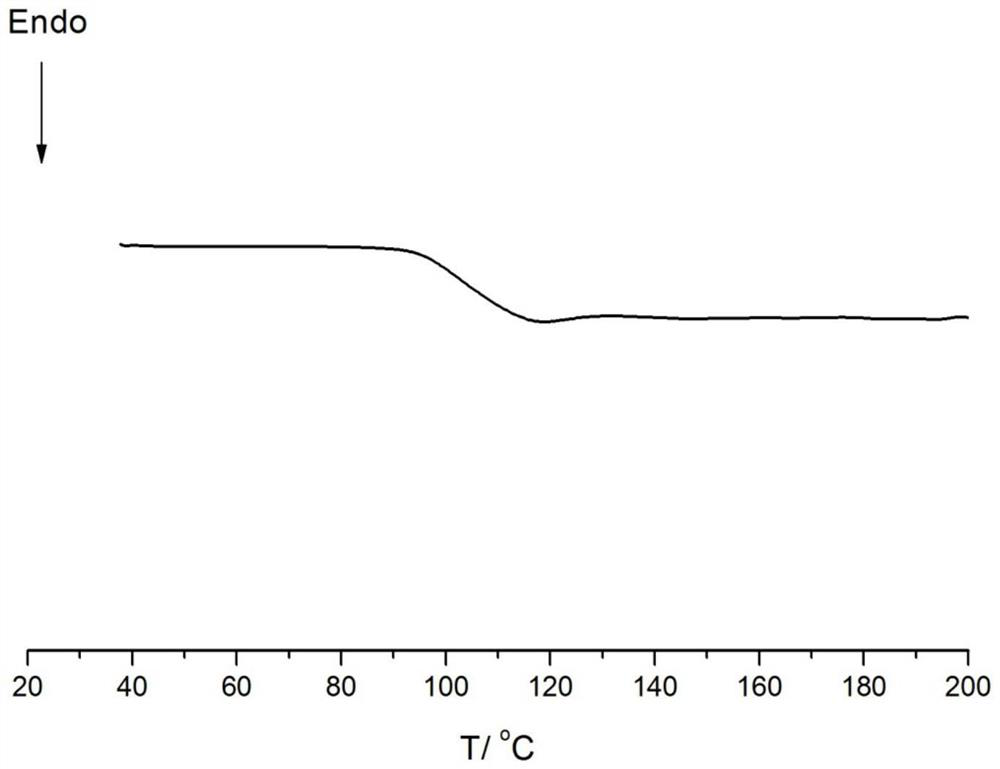
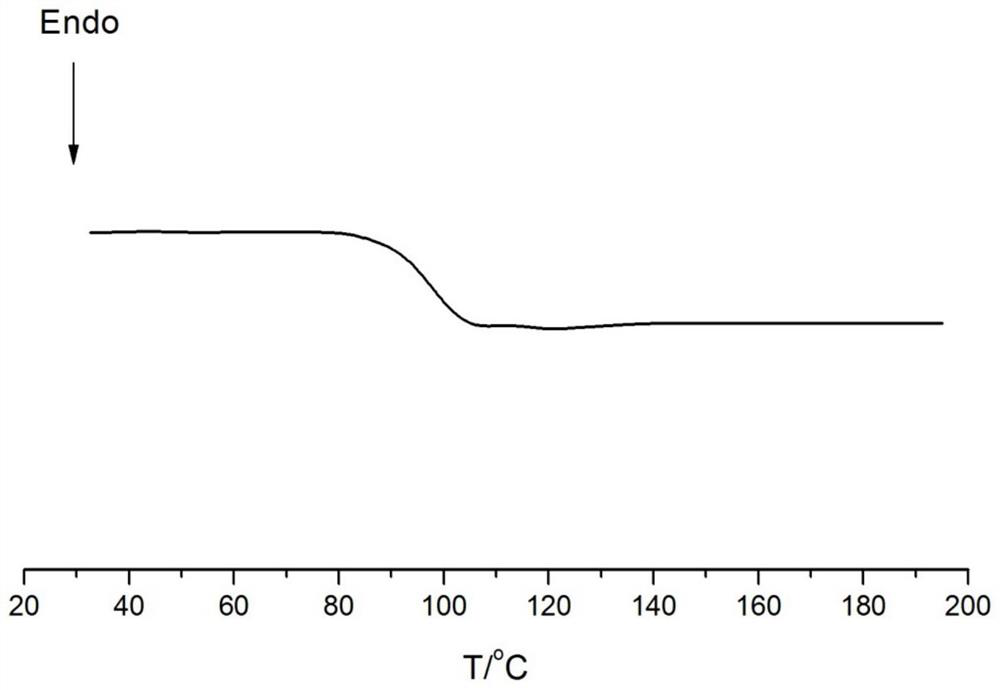
[0077] Embodiments 15-18 provide the preparation method of cycloolefin copolymer and the prepared copolymer. The polymerization conditions are respectively that monomer a is ethylene; monomer b is norbornene, concentration: 2mol / L; monomer c is 5 -Hydroxy-2-norbornene (Example 16-18), concentration: 0.5mol / L; Monomer d, concentration is 0.2mol / L (Example 16-18); Polymerization temperature: 70°C, polymerization pressure: 0.5MPa, polymerization time: 1h, catalyst dosage: 5μmol. Its specific implementation is the same as Example 1, and the difference is as shown in table 3. In addition, the polymer weight that embodiment 15-18 obtains, polymer glass transition temperature, the content of monomer b / c in the polymer, and Polymer melt index is listed in the following table 3, wherein the DSC collection of illustrative plates of embodiment 15 is as Figure 4 As shown, a Tg peak can be observed without a crystalline peak.
[0078] table 3
[0079]
[0080] Embodiments 19-22 pr...
Embodiment 23
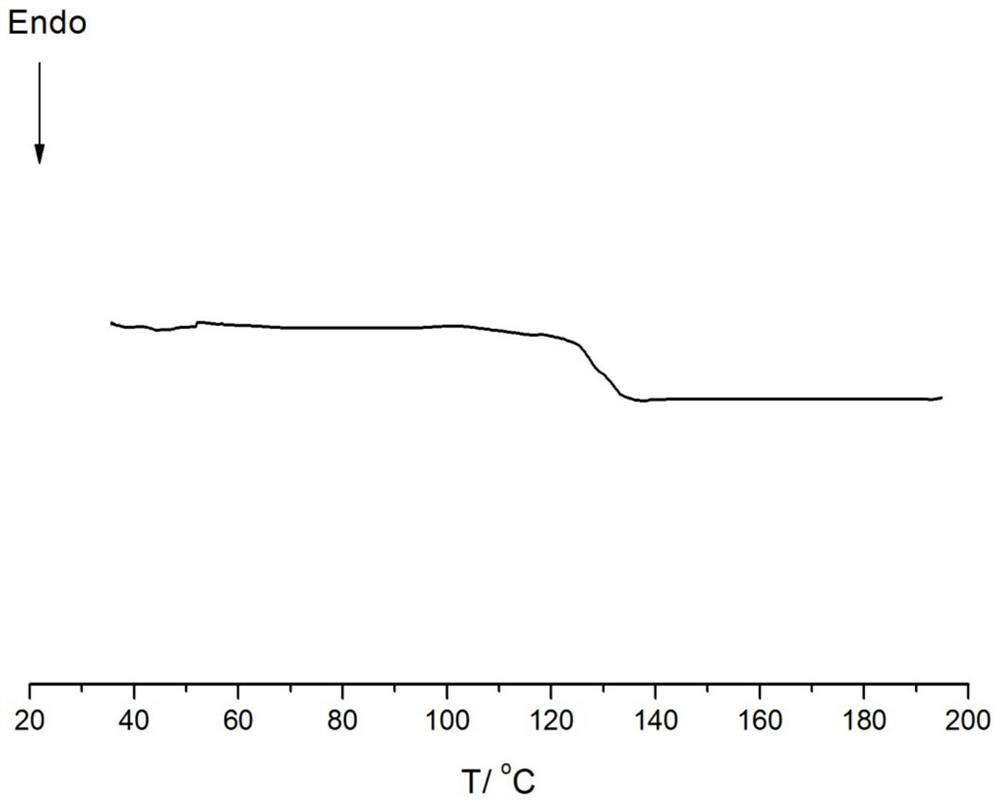
[0085] Embodiments 23-26 provide the preparation method of cycloolefin copolymer and the prepared copolymer. The polymerization conditions are respectively that monomer a is ethylene; monomer b is norbornene, concentration: 1mol / L; monomer c is 5 -Hydroxy-2-norbornene, concentration: 0.2mol / L; polymerization temperature: 70°C, polymerization pressure: 0.5MPa, polymerization time: 1h, catalyst dosage: 5μmol. Its specific embodiment is the same as Example 1, and difference is as shown in table 5, in addition, the polymer weight that embodiment 8-14 obtains, polymer glass transition temperature, the content of monomer b / c in the polymer, and Polymer melt index is listed in the following table 5, wherein the DSC collection of illustrative plates of embodiment 23 is as Image 6 As shown, a Tg peak can be observed without a crystalline peak.
[0086] table 5
[0087]
[0088]
[0089] According to the performance tests of the copolymers prepared by different monomers a, b, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com