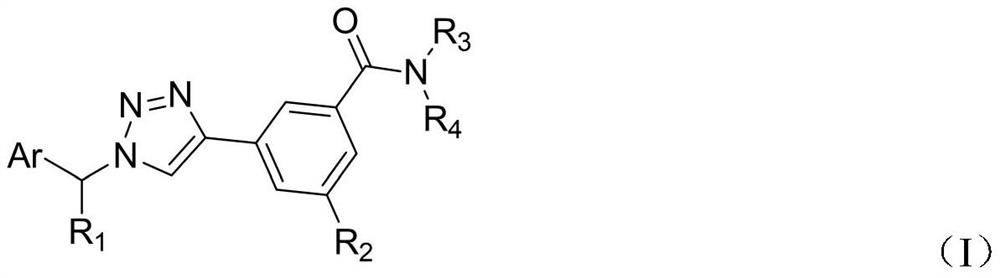
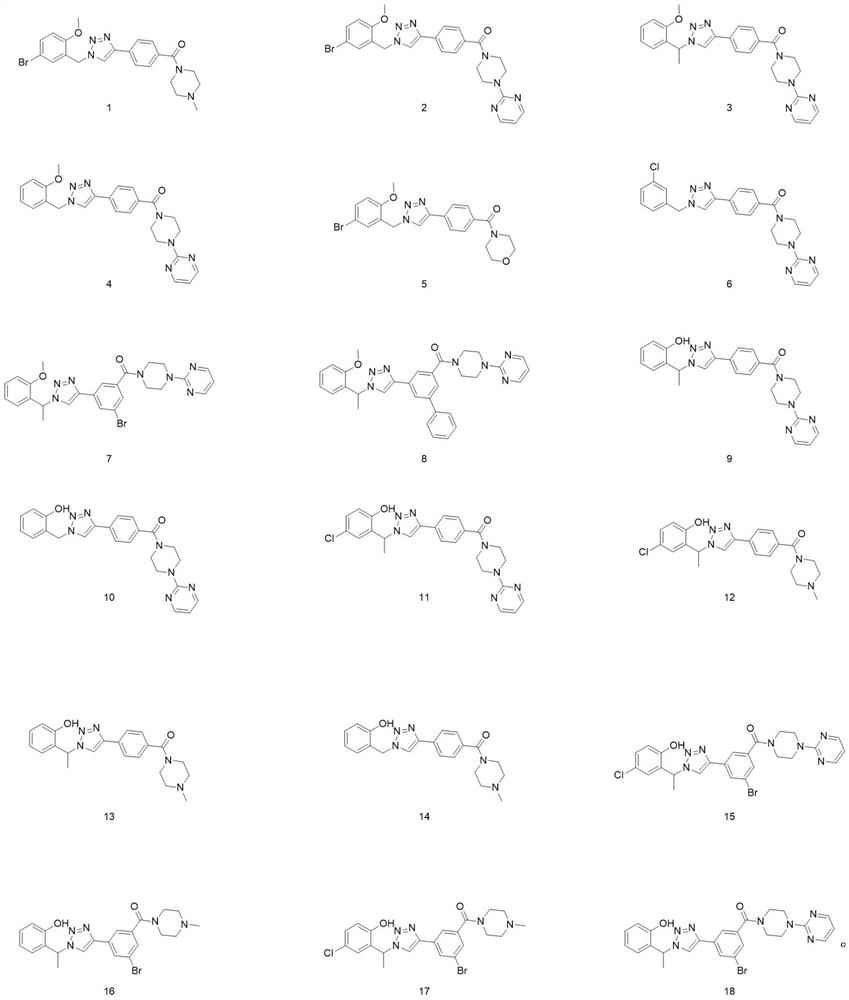
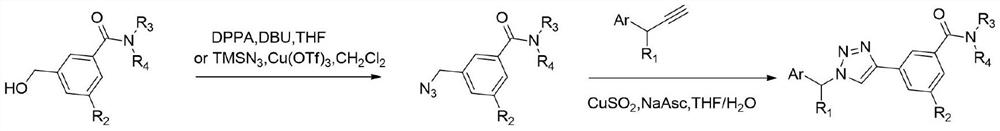
Triazole compound, preparation method and application of triazole compound in preparation of medicine for preventing and treating cancer
A compound and drug technology, which is applied in the preparation of anticancer drugs. In the field of triazole compounds, it can solve the problems of high toxicity and side effects of drugs, and achieve the effect of alleviating the side effects and effectively inhibiting the activity of LSD1 protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of compound 1
[0030]
[0031] The specific preparation method is:
[0032] 1) Preparation of compound (1-1):
[0033]
[0034] Dissolve 4-ethynylbenzoic acid (438mg, 3.00mmol) in DMF (15mL), add EDCI (863mg, 1.50mmol), HOBt (486mg, 1.20mmol), DIPEA (1.16g, 9.00mmol) N-methyl Basepiperazine (300mg, 3mmol), react at room temperature for 8h. The reaction solution was washed with saturated brine, extracted with ethyl acetate, the organic phase was dried and concentrated, and purified by column chromatography (dichloromethane:methanol=20:1) to obtain compound 1-1 (white solid, 554mg, 81%) .
[0035] The NMR test result of compound 1-1 is:
[0036] 1 HNMR (400MHz, CDCl 3 )δ7.51(d, J=8.3Hz, 1H), 7.35(d, J=8.3Hz, 1H), 3.78(s, 1H), 3.40(s, 1H), 3.14(s, 1H), 2.47( s,1H),2.30(s,2H).δ13C NMR(100MHz,CDCl 3 )δ169.6, 136.0, 132.3, 127.2, 123.7, 82.9, 78.8, 55.3, 54.8, 47.7, 42.2. HRMS (ESI) calculated for C 14 h 16 N 2 NaO + :251.1160,...
Embodiment 2
[0047] Embodiment 2: the preparation of compound 2
[0048]
[0049] The specific preparation method is:
[0050] 1) Preparation of compound (2-1):
[0051]
[0052] The specific preparation method is: similar to the preparation method of compound 1-1, the difference is that one of the raw materials used is 5-(piperazin-1-yl)pyrimidine, and an equimolar substitution of compound (1-1) in the preparation method of N-methylpiperazine.
[0053] The NMR test result of compound 2-1 is:
[0054] 1 H NMR (400MHz, CDCl 3 )δ8.29(d, J=4.9Hz, 2H), 7.52(d, J=7.9Hz, 2H), 7.37(d, J=8.0Hz, 2H), 6.51(t, J=4.8Hz, 1H) ,4.03–3.66(m,6H),3.54–3.38(m,2H),3.16(s,1H). 13 CNMR (100MHz, CDCl 3 )δ169.8, 161.5, 157.8, 135.8, 132.3, 127.2, 123.8, 110.6, 82.8, 79.0, 47.5, 44.0, 43.5, 42.2. HRMS (ESI) calculated for C 17 h 16 N 4 NaO + :315.1222,found 315.1224.
[0055] 2) Preparation of compound 2:
[0056] The specific preparation method is: similar to the preparation method of compound ...
Embodiment 3
[0059] Embodiment 3: the preparation of compound 3
[0060]
[0061] The specific preparation method is: similar to the preparation method of compound 1, the difference is that one of the raw materials used is compound 2-1, and N-methylpiperazine in the preparation method of compound (1-1) is replaced by equimolarity; One of the raw materials used, methoxydimethylbenzylmethanol, replaces 5-bromo-2-methoxybenzylmethanol in the preparation method of compound (1-2) by equimolarity.
[0062] The NMR test result of compound 3 is:
[0063] 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=4.7Hz, 2H), 7.86(d, J=8.0Hz, 2H), 7.73(s, 1H), 7.47(d, J=8.0Hz, 2H), 7.31(td, J=7.9,1.7Hz,1H),7.20(dd,J=7.7,1.6Hz,1H),6.89-6.97(m,2H),6.52(t,J=4.7Hz,1H),6.22(q,J =7.1Hz,1H),3.82(s,9H),3.51(s,2H),1.97(d,J=7.1Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ170.4, 161.6, 157.8, 156.4, 146.3, 134.8, 132.6, 129.8, 128.1, 127.8, 126.9, 125.7, 121.0, 119.5, 110.9, 110.6, 55.6, 54.6, 47.6, 44.1, 42.HR, 6, 20. )
[0064] c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com