Compositions comprising 15-hepe and/or 15-hetre and methods of treating or preventing cardiometabolic disease, metabolic syndrome, and/or related diseases
A 15-HEPE, metabolic syndrome technology, applied in metabolic diseases, drug combinations, urinary system diseases, etc., can solve problems such as increased risk of heart disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
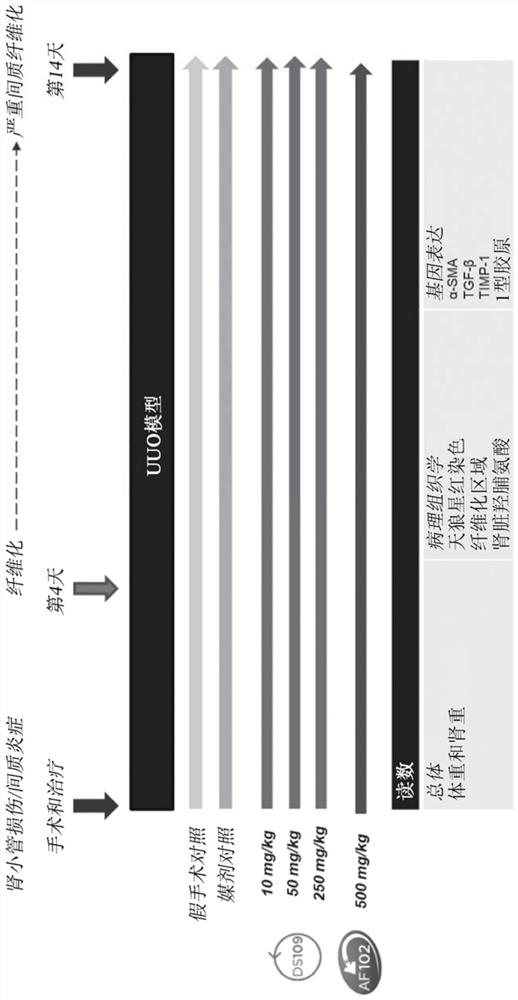
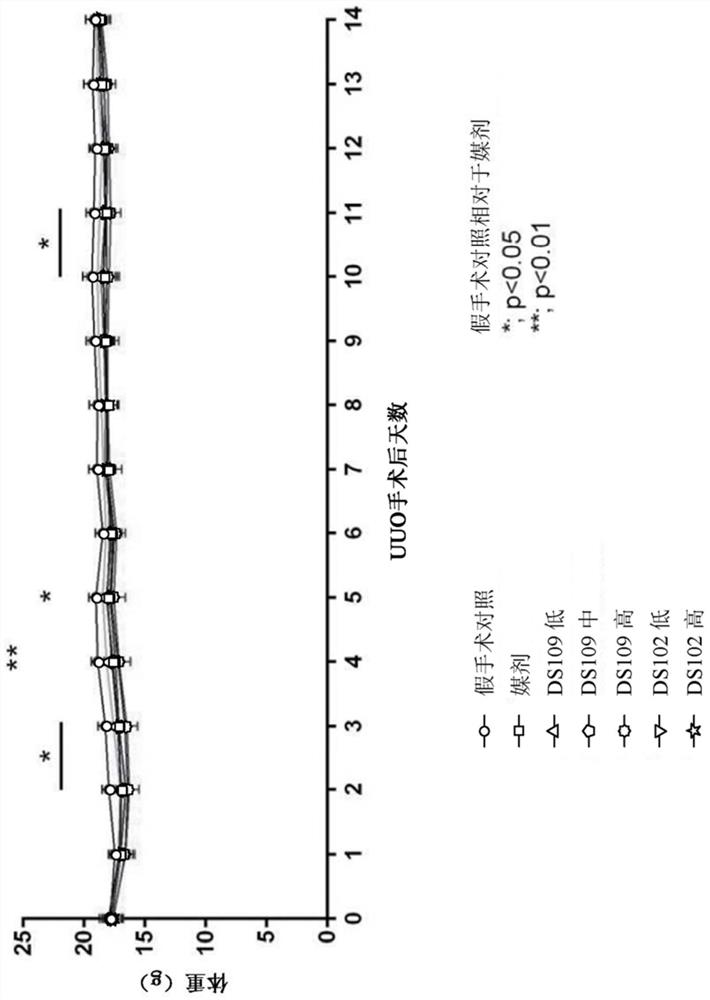
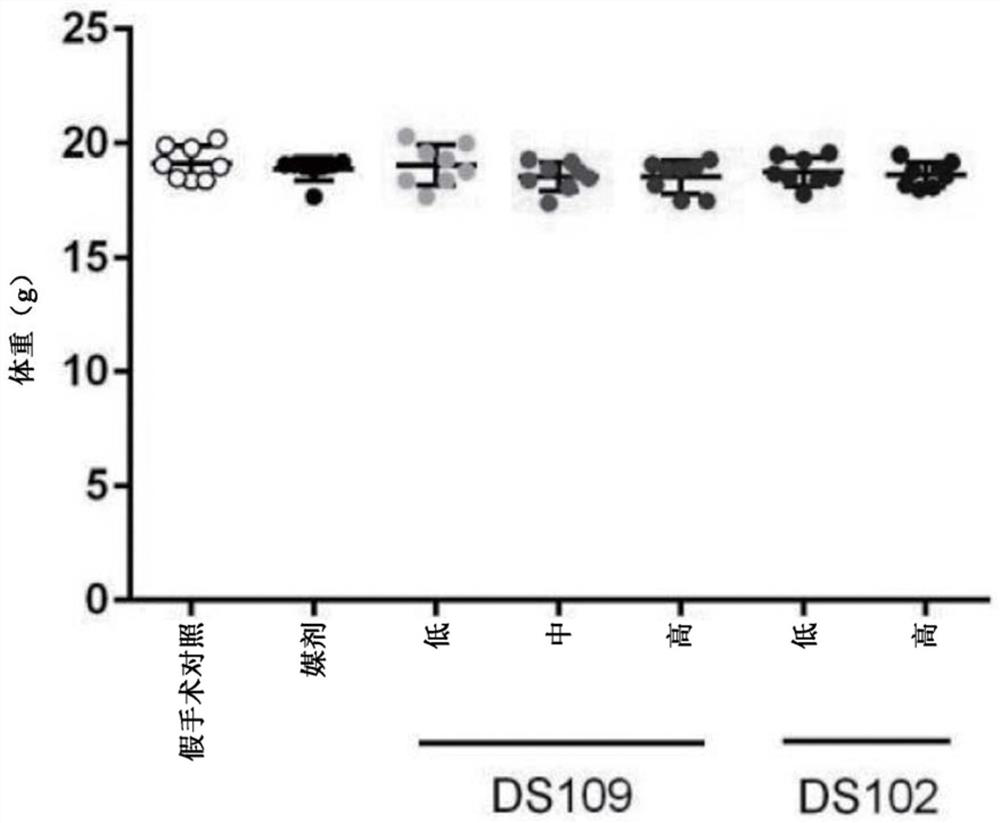
[0358] Example 1: Renal interstitial fibrosis caused by unilateral ureteral obstruction
[0359] The purpose of this study was to examine the effects of DS109(15-HETrE) and DS102(15-HEPE) on UUO-induced renal interstitial fibrosis.
[0360] figure 1 The study design is depicted from surgery and treatment to study day 14.
[0361] 1.1 Materials and methods
[0362] Test substances: The test substances in this study are DS109 (15-HETrE) and DS102 (15-HEPE). To prepare dosing solutions for each substance, DS109 was first weighed and then dissolved in a vehicle of 0.5% hydroxypropylmethylcellulose (HPMC), and DS102 was diluted in a vehicle of 0.5% HPMC.
[0363] UUO surgery: On study day 0, mice underwent UUO surgery under pentobarbital sodium anesthesia. The mice were first shaved, and then the abdomen was incised to remove the mouse's left ureter from the abdomen. Using 4-0 nylon sutures, the ureter was ligated at two points. The peritoneum and skin of the mouse were then...
example 2
[0413] Example 2: Biliary Ligation (BDL) Study in Cholestatic Liver Disease and / or Hepatic Fibrosis
[0414] The aim of this study was to examine the effect of DS012 on BDL-induced cholestasis.
[0415] Figure 9 The study design is depicted from surgery and treatment to study day 14.
[0416] 1.1 Materials and methods
[0417] Test substance: The test substance in this study is DS102. To prepare dosing solutions for each substance, DS102 was diluted in a vehicle of 0.5% hydroxypropylmethylcellulose (HPMC).
[0418] BDL surgery: On study day 0, BDL surgery was performed under pentobarbital (Kyoritsu Seiyaku, Japan) anesthesia. Firstly, the mouse hair was shaved, the abdominal cavity was cut open, and the common bile duct was ligated twice with 7-0 surgical silk. The peritoneum and skin of the mouse are closed with sutures, and the mouse is transferred to a clean cage (eg, a rest cage) until recovery from anesthesia. The common bile ducts of sham-operated mice were expose...
example 3
[0466] Example 3: Effect of DS102 on TGF-beta receptor, signaling and induced fibrotic proteins
[0467] The aim of this study was to investigate the effects of 15-HEPE and 15-HEPE EE on TGF-β receptor expression, TGF-β-induced intracellular signaling, and pro-fibrotic epithelial-mesenchymal transition proteins.
[0468] 1.1 Materials and methods
[0469] Cytotoxicity test: The cytotoxicity of 15-HEPE free acid and ethyl ester was tested in different liver (hepatocarcinoma) cell lines to understand the concentration range in the test system.
[0470]Transcriptional activity: A promoter (luciferase) assay was performed to measure TGFβ-induced transcriptional activation following 15-HEPE administration.
[0471] Identification of sucrose-induced 15-HEPE-induced microdomain translocation of TGF-β receptors using sucrose gradient ultracentrifugation and confocal microscopy. TGF-β receptor activation was performed in the plasma membrane of Mv1Lu cells (mink lung epithelial cells)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com