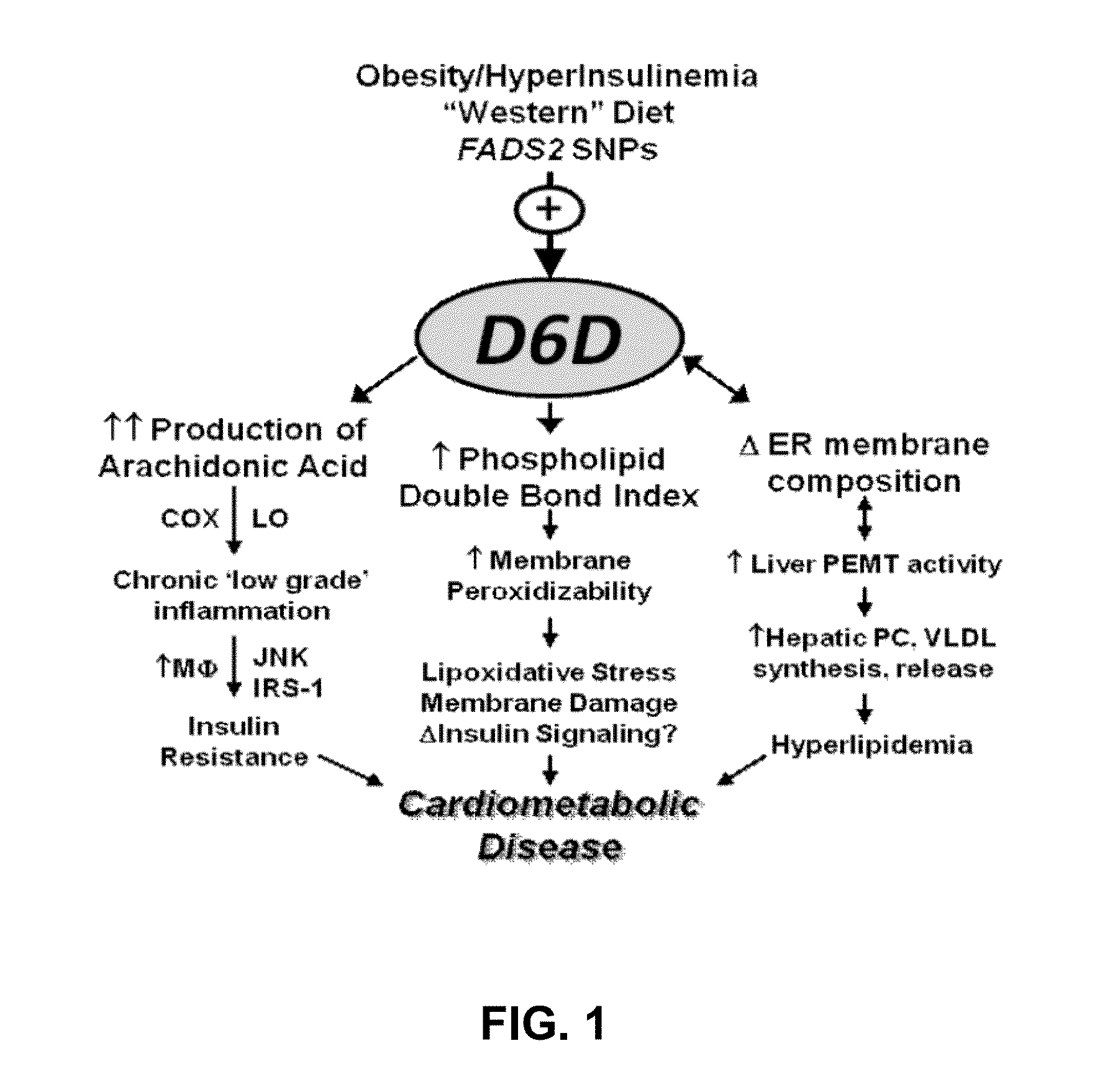
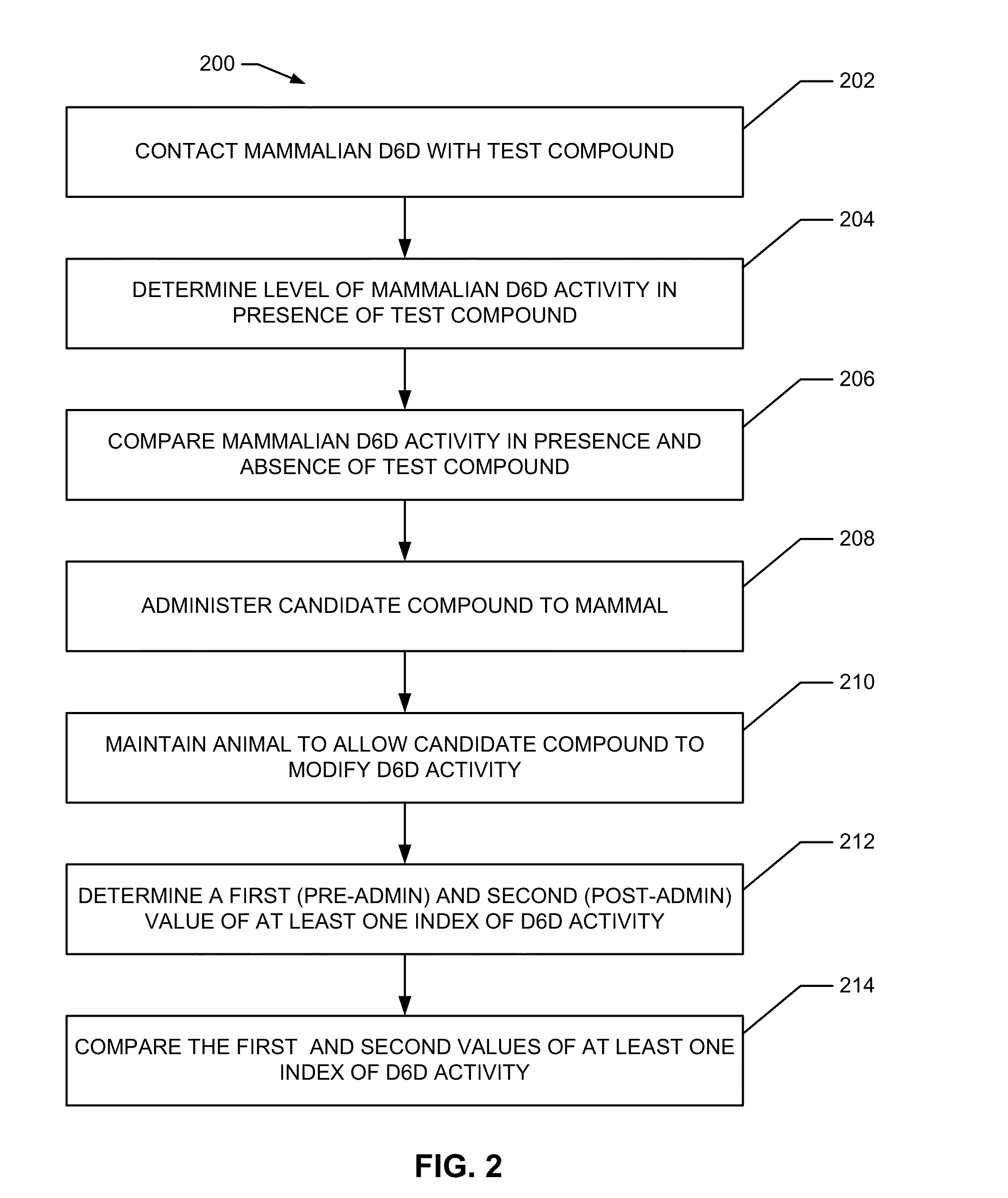
Inhibition of delta-6 desaturase for the treatment of cardiometabolic disease
a cardiometabolic disease and desaturase technology, applied in the field of desaturase inhibition of delta6 desaturase for the treatment of cardiometabolic disease, can solve the problems of complex and risky approach, imbalance in the relative distribution of species with different, or even opposing, pathogenic potentials, and the targeting of select aa metabolism enzymes as an anti-inflammatory treatment of metabolic disease and its complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of D6D Ameliorates Cardiometabolic Disease in Mice
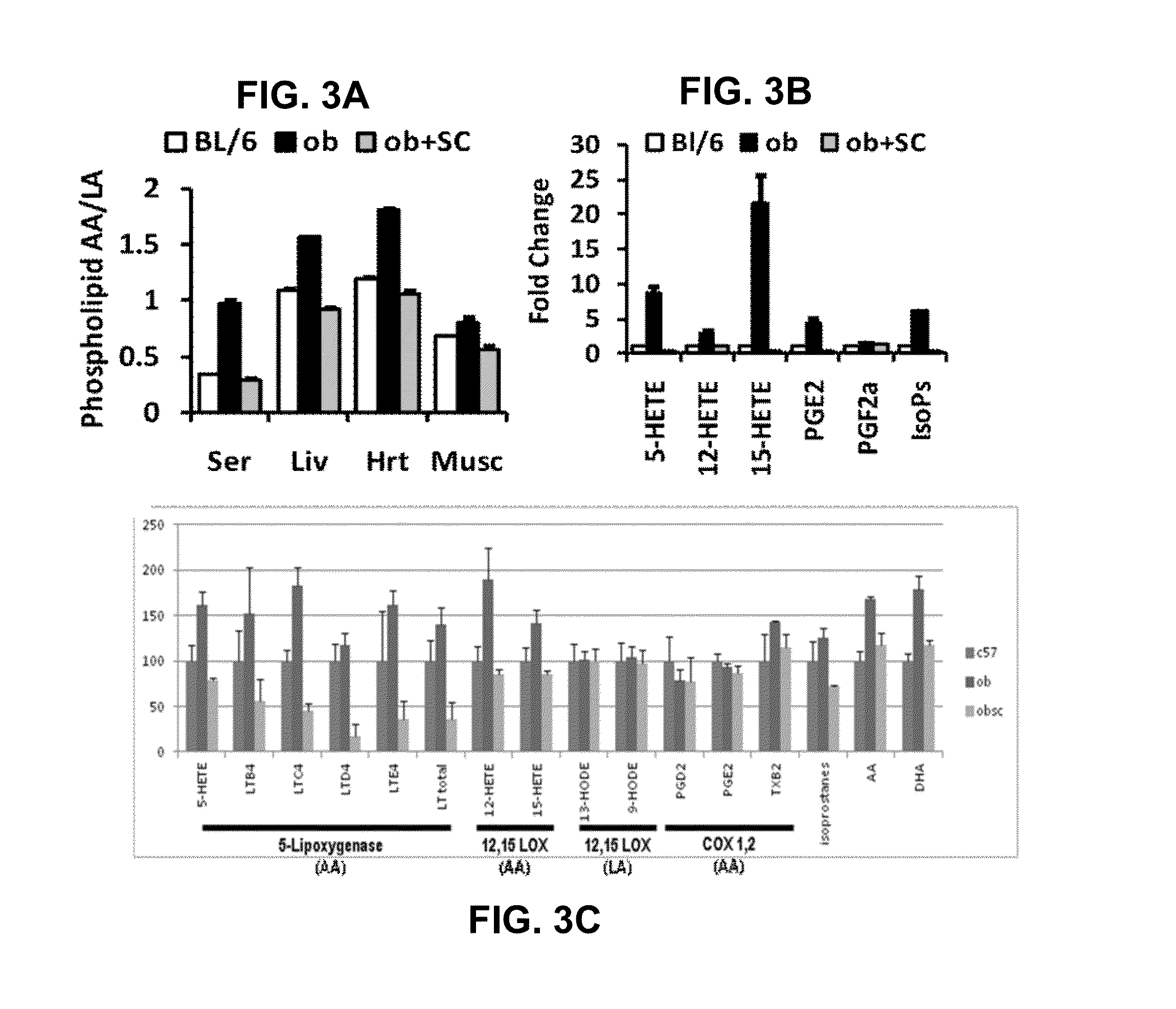
[0083]To assess the effects of D6D inhibition on structures and processes related to cardiometabolic diseases and disorders, the following experiments were conducted. Animal studies have reported evidence of increased D6D activity in rodent models of CMD (e.g., increase in AA / LA in tissue phospholipids), making them reasonable models for studying the role of D6D in its pathogenesis. To probe the effect of D6D directly, the compound SC-26196, a selective D6D inhibitor was orally administered by admixing the SC-26196 in the food mixtures of the rodent models of CMD at a dosage of about 100 mg / kg / day for four weeks. The rodent models of CMD used in these experiments included a murine model of hyperphagic obesity (leptin-deficient ob mice) and a diet-induced insulin resistance model (normal C57Bl / 6 mice fed a high-fat “Western” diet) for 8 weeks prior to participation in any experiments.
[0084]Initially, the effects of D6D inhi...
example 2
Generation of Fads2 Transgenic Mice
[0089]To develop and characterize a transgenic mouse with global over-expression of the D6D gene, Fads2, the following experiments were conducted.
[0090]A full length mouse Fads2 cDNA sequence (1.6 kb) was cloned into the EcoRV and Notl site of the pcDNA-3.1(+) mammalian expression vector as illustrated schematically in FIG. 15. In this vector, Fads2 was placed downstream of a CMV promoter and upstream of a polyadenylation sequence (BGHpa) and neomycin resistant gene. To generate transgenic mice over-expressing Fads2, a purified ˜4.6 kb Nrul / Bst1107I fragment containing the CMV-Fads2 expression cassette, was microinjected into pronuclei of fertilized FVB / N mice.
[0091]Transgenic positive founder mice were identified by Southern blot and PCR amplification of genomic DNA isolated from the tail. An image of a representative Southern blot comparing wild type (WT), transgenic (TG), and a negative control (NEG) is provided in FIG. 16.
[0092]Independent foun...
example 3
Overexpression of D6D Increases Fasting Glucose and Impairs Glucose Tolerance
[0094]To assess the consequences of D6D overexpression in processes and structures related to cardiometabolic disease and disorders, the following experiments were conducted. A line of mice with transgenic overexpression of the D6D gene (fads2) were developed using the methods summarized in Example 2
[0095]Phenotyping studies of the first-fifth generation of fads2-TG mice at 3-4 months of age were performed to characterize a number of structures related to cardiometabolic disease and disorders that may be related to D6D hyperactivity. Corresponding measurements were performed on wild-type (WT) littermates to assess differential effects due to D6D overexpression. As summarized in FIG. 21, fads2 overexpression impaired glucose tolerance, increased AA / LA ratio in serum, liver, heart, and muscle tissues, and increased fads2 mRNA expression to levels comparable to ob mice (ob) or Western diet-induced cardiometabo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com