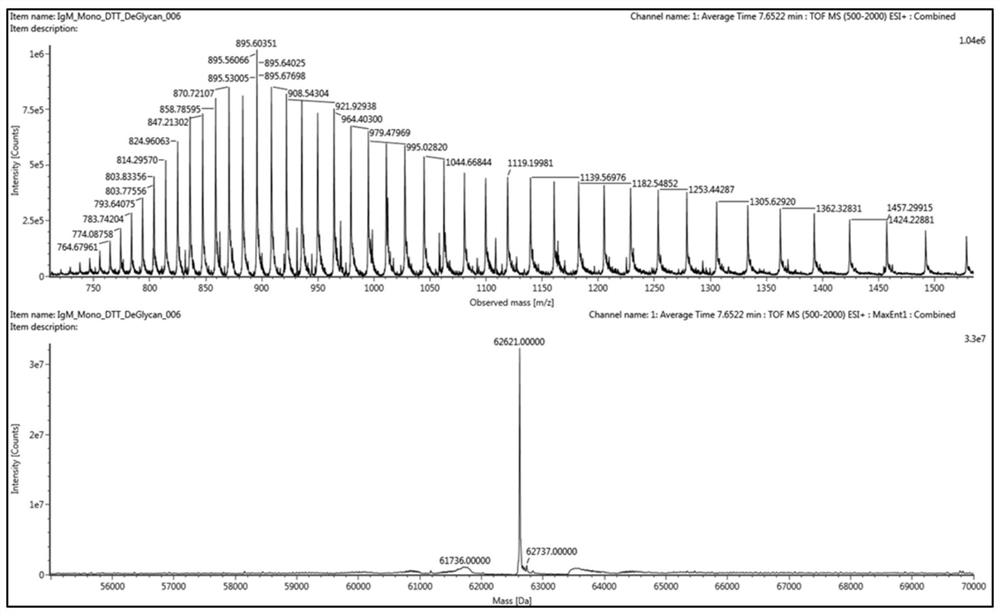
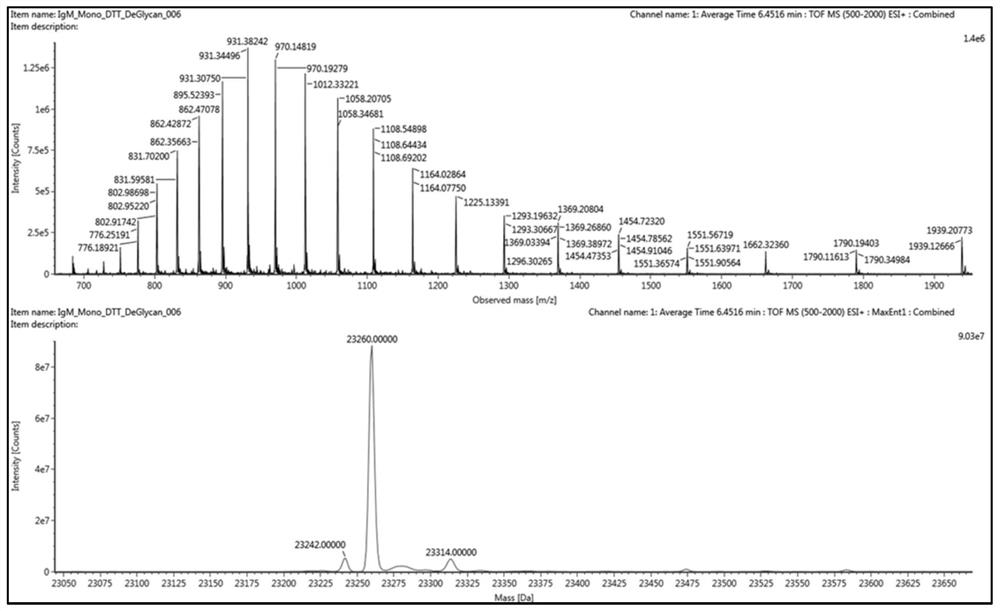
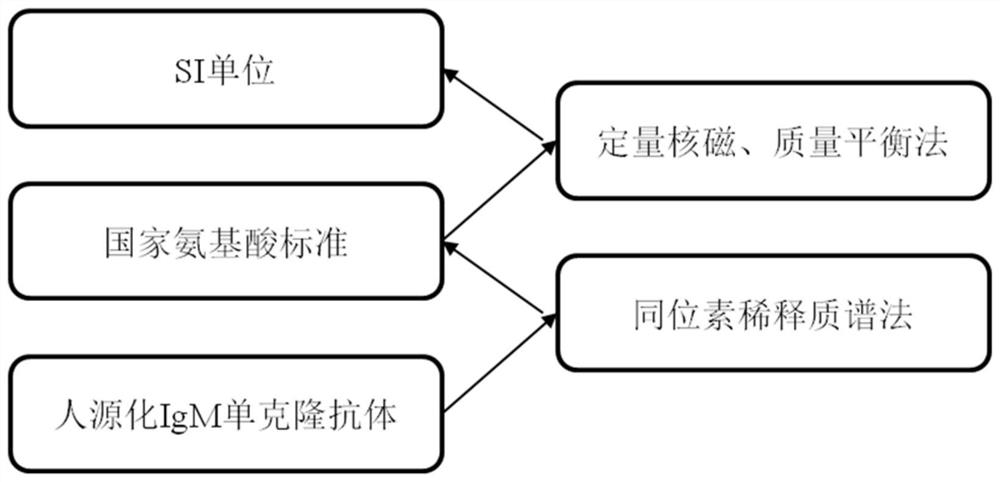
Humanized IgM monoclonal antibody standard substance and preparation method thereof
A monoclonal antibody and standard substance technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as large molecular weight and complex structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] For acute infectious diseases caused by sudden outbreaks of new viruses, taking the 2019-2020 novel coronavirus pandemic as an example, the development process of the 2019-nCoV new coronavirus N protein human IgM monoclonal antibody solution standard material is demonstrated, and the specific design, The experimental process is as follows:
[0067] 1. Screening and protein expression of human IgM monoclonal antibody against N protein of 2019-nCoV novel coronavirus
[0068] According to the sequence of the novel coronavirus N gene (position 28274-29533) published in NCBI GenBank, the N complete protein is expressed, and the phage display library constructed after immunizing animals with the N protein (the storage capacity is about 10 9 ) to carry out antibody screening on the N antigen to obtain the IgG prototype antibody with a certain affinity (affinity threshold Kd-7 ). The IgG prototype antibody was subjected to affinity maturation, and then the optimized variable r...
Embodiment 2
[0164] For common important infectious diseases, taking AIDS as an example, IgM monoclonal antibody standard substances can also be developed according to the technical scheme of the present invention:
[0165] At present, there are no certified reference materials for AIDS IgM antibodies in China. There are two main reasons:
[0166] 1. It is impossible to obtain and process the serum of AIDS patients as a standard product under daily conditions;
[0167] 2. Even if dozens of AIDS patients’ serum can be obtained, the components in it are very complex, containing only 1% or even less than 0.1% of anti-HIV polyclonal IgM antibodies, which is difficult to accurately characterize and quantify to make it a certified standard substance.
[0168] Therefore, the scheme in Example 1 was used to screen IgG antibodies against AIDS antigens, and humanized transformation and variable region grafting were carried out to obtain humanized IgM antibodies, which were expressed and purified in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com