Adenosomes
An adenovirus and recombinant adenovirus technology, applied in the field of adenoviruses, can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
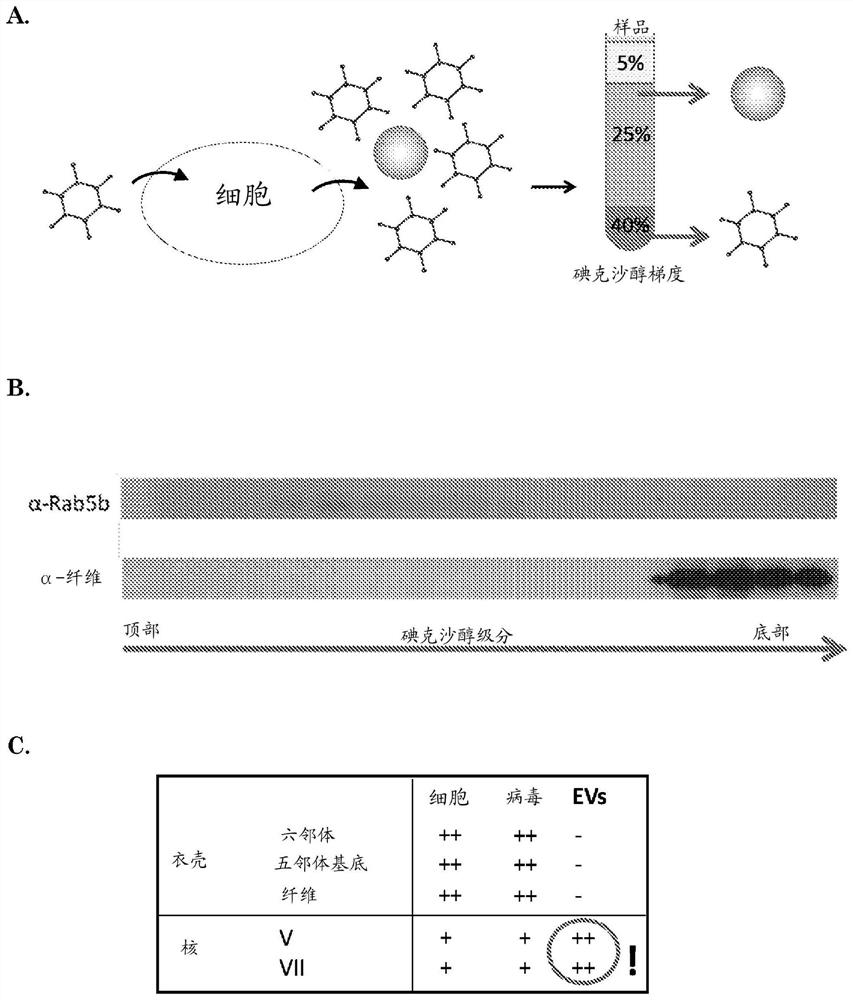
[0182] Example 1: Detection of Adenoviral Nucleoproteins in EVs by Mass Spectrometry
[0183] GS184 cells (primary glioblastoma cells obtained from Erasmus MC, Department of Neurosurgery) were plated in ten T175 flasks for 24h and then infected with wild-type HAdV-5. Cells were treated as a monolayer in NS medium (DMEM-F12+fibroblast growth factor+endothelial growth factor+heparin+B27+1% penicillin-streptomycin) with extracellular matrix (ECM 1:100 in dPBS, Cultrex , Sanbio) coating. Cells were infected with wild-type human adenovirus type 5 (HAdV-5) at an MOI of 5 infectious particles / cell. After 2 hours the medium was replaced with NS medium. Supernatants and cells were collected 40 hours after infection. From cell culture supernatants using an iodixanol-based density gradient (ultracentrifugation at 192,000g for 4 hours) as described in De Vrij, 2013, Nanomedicine (Lond), 8(9):1443-58 EV. The iodixanol gradient consisted of three layers: 40% iodixanol as the bottom l...
Embodiment 2
[0184] Example 2: Detection of oncolytic adenoviral DNA in EVs by PCR and EVs are able to infect cells
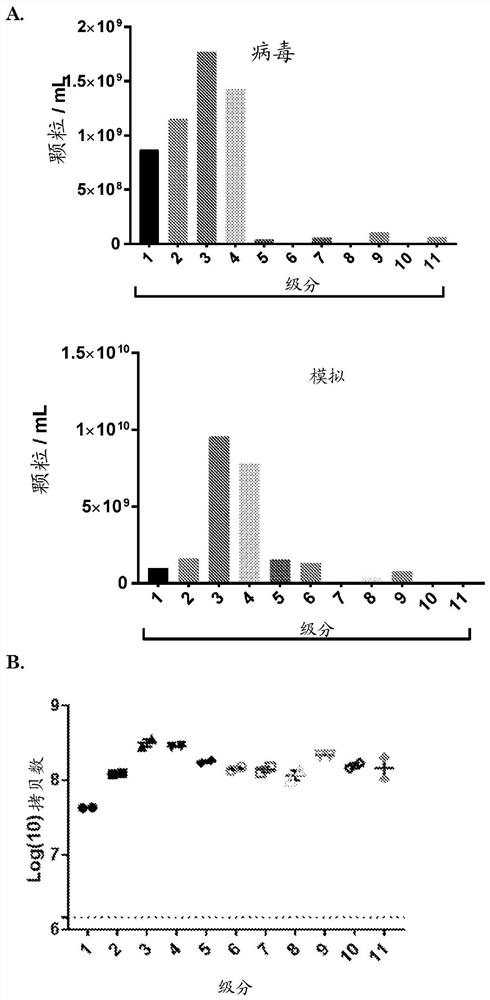
[0185] GS756 cells were plated in two T175 flasks 24 h before infection with conditionally replicating adenovirus (CRAd) Ad.5.d24.RGD.GFP (MOI = 1 infectious particle / cell). (Virus details including genomic construction are provided in Balvers, 2014, Viruses, 6(8), 3080-3096.) After 72 hours, the supernatant was collected for EV isolation. Low speed centrifugation was performed to remove cells and cell debris (150 x g for 5 min plus 3000 x g for 20 min) followed by ultracentrifugation at 100.000 x g for 70 min to pellet EVs and viral particles. The pellet was subjected to iodixanol density gradient centrifugation and the iodixanol fraction was isolated as described above.
[0186] To determine the amount of EVs within each fraction, samples were prepared for EV-Quant analysis. For this, the samples were incubated with a red fluorescent dye (rhodamine at a final concentr...
Embodiment 3
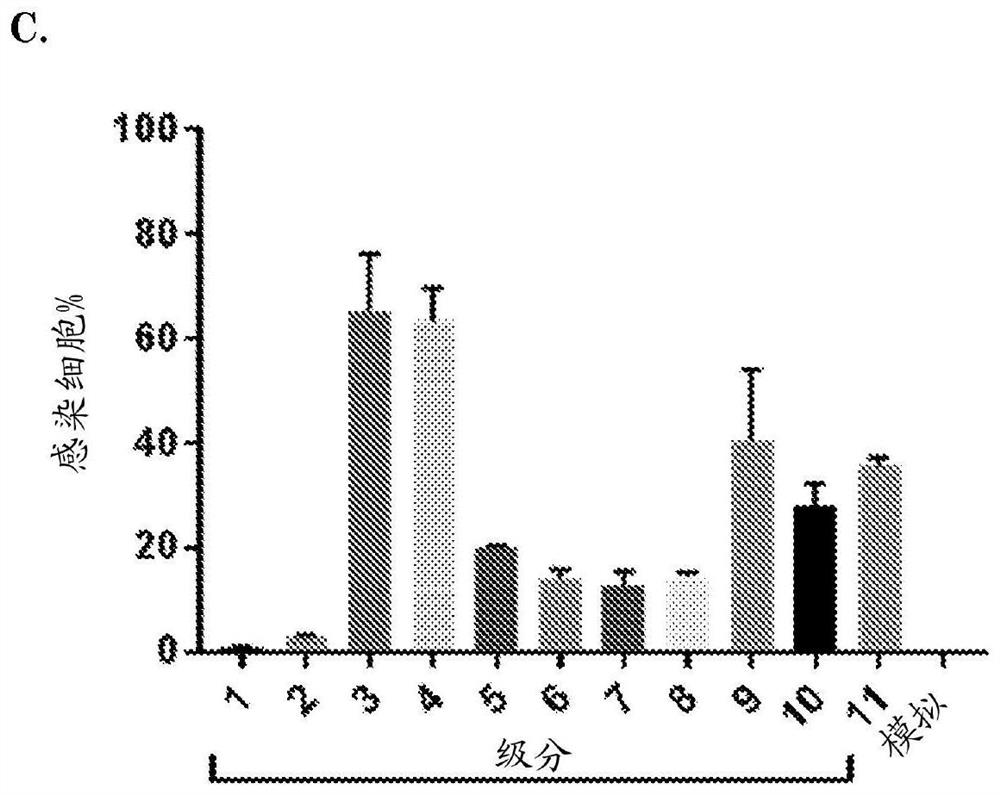
[0189] Example 3: EV-Quant demonstrates pV.GFP incorporation into EVs following infection of cells with pV.GFP virus
[0190] Cells (GS184, GS562, A549 and HER911 ) were plated on 48-well plates for 24 hours and then infected with adenovirus (pV.GFP) with green fluorescent protein attached to the pV protein. Infect cells with an MOI of 50 viral particles / cell in 100 μL of medium / well (NS medium for GS184 and GS562 and serum medium for HER911 / A549). (Serum medium: Dulbecco's modified Eagle's medium+10% fetal bovine serum (FBS)+1% penicillin-streptomycin). For each cell line, the medium was changed to NS medium, DMEM only medium and serum medium after 2 h. (Serum medium was precleared from bovine EVs by ultracentrifugation at 100,000 xg for 16 h). For all cell lines, each media condition was used in triplicate. Supernatants were collected 64 hours post-infection and centrifuged at 500xg for 10 minutes to remove cell debris. Perform EV-Quant analysis on 143 μL of all supern...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com