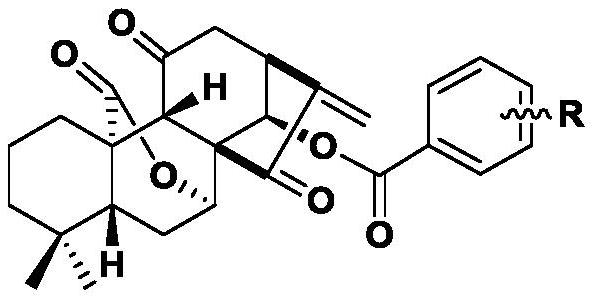
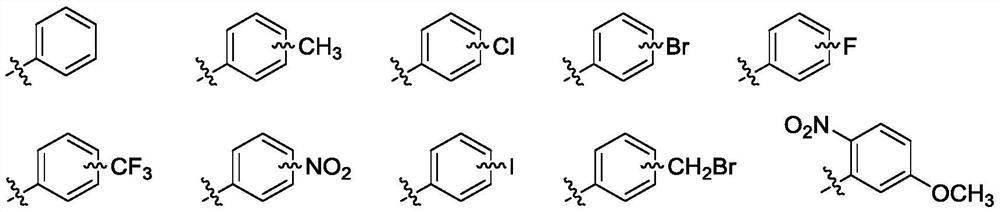
11,20-Dicarbonyl Jiyuan Rubescensin A 14-O-benzoate derivative and its preparation method and use
A technology of oridonin A and benzoate, applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of poor water solubility, difficult oral absorption, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
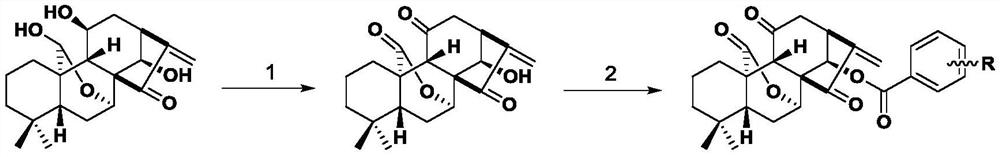
Embodiment 1
[0021]
[0022] Weigh 300 mg of JOA, add 11 mL of acetone to dissolve it completely, and add 0.75 mL of Jones reagent (6 mL of acetone for dilution) under stirring conditions. Spin to dryness, add 30 mL of ethyl acetate to the reaction system to dilute, wash the reaction system with saturated NaCl solution three times, reverse-extract with water once with ethyl acetate, combine the organic phases and dry with anhydrous magnesium sulfate, concentrate, and perform column chromatography. After purification, 270 mg of the target compound 11,20-dicarbonyl Jiyuan oridonin A was obtained with a yield of 74%. 1 H NMR (400MHz, DMSO-d 6 )δ6.14(s,1H),6.08(d,J=2.9Hz,1H),5.79(s,1H),4.79(dd,J=3.9,1.7Hz,1H),3.74–3.71(m,1H) ), 3.10(d, J=8.3Hz, 1H), 2.96(s, 1H), 2.92–2.83(m, 2H), 2.66(dd, J=16.3, 8.4Hz, 1H), 2.55(s, 1H) ,1.76(ddd,J=14.4,7.6,4.0Hz,1H),1.59(dp,J=12.6,5.1,4.3Hz,2H),1.36(d,J=11.6Hz,2H),1.09(td,J =11.4,10.0,5.2Hz,1H),0.98(td,J=14.0,4.4Hz,1H),0.81(s,3H),0.72(s,3H). 13 C NMR (...
Embodiment 2
[0024] Compound 1a
[0025] Weigh 200mg 11,20-dicarbonyl Jiyuan Rubescensine A and dissolve it in 10mL of dichloromethane, add 92mg of benzoic acid under stirring, then add catalyst 1-(3-dimethylaminopropyl group in turn )-3-ethylcarbodiimide hydrochloride (EDCI) 144 mg and 4-dimethylaminopyridine (DMAP) 9 mg. After the completion of the reaction monitored by thin-layer chromatography, 30 mL of dichloromethane was added to dilute the reaction system, and the reaction system was washed three times with saturated sodium bicarbonate solution, the aqueous layers were combined and back-extracted once, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, column Chromatography gave the product as a white solid in 85% yield. 1 H NMR (400MHz, DMSO-d 6 )δ7.62(d,J=7.8Hz,2H),7.44(d,J=7.5Hz,1H),7.29(t,J=7.5Hz,2H),6.04(s,1H),5.68(s, 1H), 4.79(s, 1H), 4.60(d, J=3.5Hz, 1H), 3.10(s, 2H), 3.07(s, 1H), 2.66(d, J=13.8Hz, 2H), 2.59( t, J=8.3Hz, 1H), 2.26(s, 1H...
Embodiment 3
[0027] Compound 2a
[0028] Substitute 115 mg of 2-methoxybenzoic acid for benzoic acid, and other operations are the same as in Example 2 to obtain a white solid product with a yield of 47%. 1 H NMR (400MHz, DMSO-d 6 )δ7.61(dd,J=7.9,1.8Hz,1H),7.58-7.55(m,1H),7.16-7.13(m,1H),7.04-7.01(m,1H),6.25(s,1H) ,5.91(s,1H),4.99(d,J=1.1Hz,1H),4.83(dd,J=4.1,1.8Hz,1H),3.77(d,J=2.5Hz,3H),3.46(d, J=8.4Hz, 1H), 3.28(s, 1H), 2.92(ddd, J=13.1, 8.0, 2.6Hz, 2H), 2.81(d, J=8.2Hz, 1H), 2.71(d, J=16.4 Hz, 1H), 1.89–1.84 (m, 1H), 1.69–1.65 (m, 1H), 1.41 (d, J=3.9Hz, 1H), 1.37 (s, 1H), 1.23 (s, 1H), 1.11 (d, J=3.8Hz, 1H), 1.09–1.07(m, 1H), 0.83(s, 3H), 0.73(s, 3H). 13 C NMR (101MHz, DMSO-d 6)δ203.48,198.47,174.02,164.49,158.56,146.22,134.56,131.10,122.73,120.25,118.25,112.78,73.80,72.65,58.81,56.30,55.71,46.55,45.32,43.16,39.76,38.55,33.83,30.49,28.16 , 23.13, 18.97, 18.15.HR-MS(ESI): Calculated for C 28 H 30 O 7. [M+NH 4 ] + :496.2335.found:496.2328.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com