Drug-loaded nano-micelle with multiple drug release functions and preparation method of drug-loaded nano-micelle
A drug-loaded nanometer and drug-release technology, which is applied in the field of drug-loaded nanomicelles and its preparation, can solve the problems of limiting the quality and industrialization of micelles, long-term toxicity research, and poor biodegradability, so as to improve the drug cell uptake rate, Effects of improving drug compliance and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
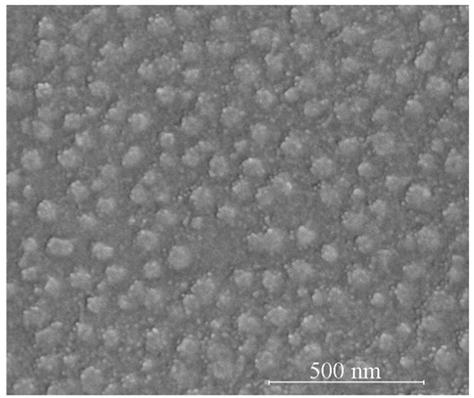
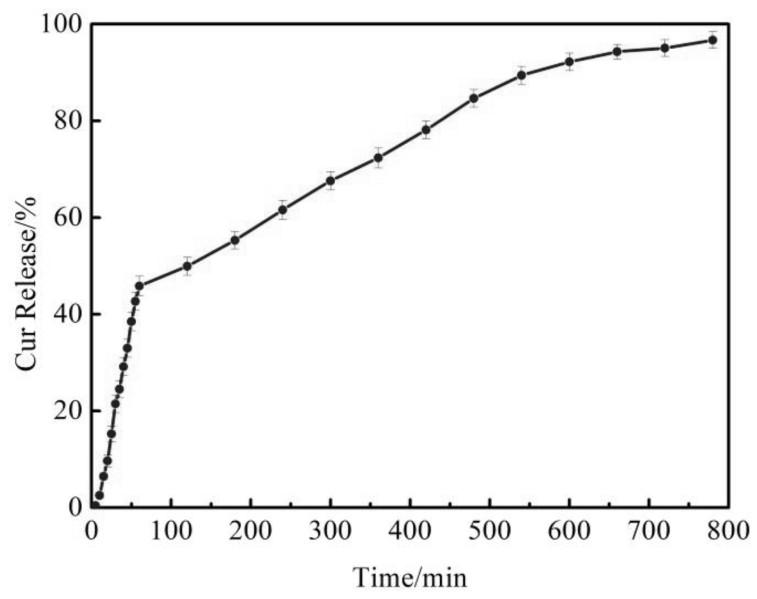
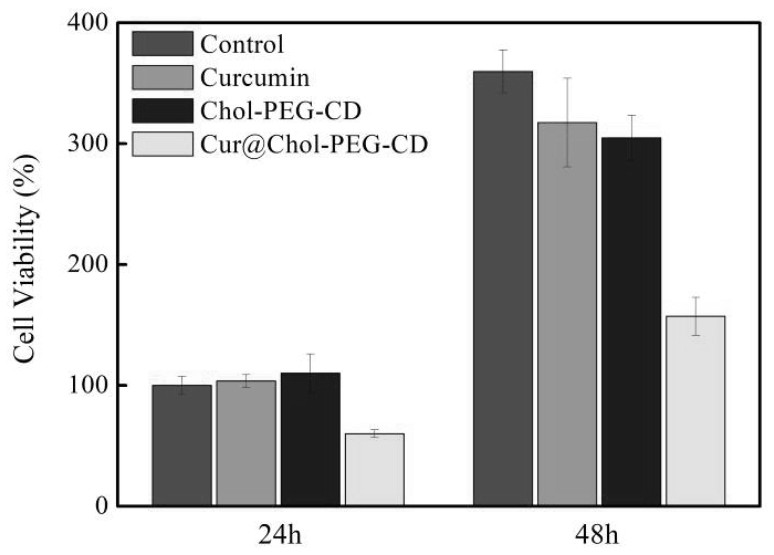
Image
Examples
Embodiment 1
[0027] (1) Carboxylation modification of β-CD: prepare a β-CD aqueous solution with a mass concentration of 1%, and mix β-CD, NaBr, and TEMPO uniformly in a molar ratio of 1:1:1 under ice bath conditions; then Add a NaClO solution with an available chlorine content of 10% at a molar ratio of 0.8:1 to β-CD; adjust the pH value of the system to 10 with 0.5M NaOH solution, and react in the dark for 5 h under ice bath conditions, then add absolute ethanol The reaction was terminated; finally, the mixture was dialyzed for 4 days and freeze-dried to obtain β-CD-COOH;
[0028] (2) Preparation of CD-PEG monomer: prepare β-CD-COOH aqueous solution with a mass concentration of 0.5%, mix β-CD-COOH, EDC and DMAP at a molar ratio of 1:2:1, and stir at room temperature 1 h; then add PEG with a molar ratio of 2.1:1 to β-CD-COOH and a relative molecular mass of 10,000 Da, stir and react at room temperature for 12 h; finally dialyze the mixture for 5 d, and freeze-dry to obtain CD-PEG monomer...
Embodiment 2
[0032] (1) Carboxylation modification of β-CD: Prepare a β-CD aqueous solution with a mass concentration of 2%, and mix β-CD, NaBr, and TEMPO uniformly in a molar ratio of 1:3:2 under ice bath conditions; then Add a NaClO solution with an available chlorine content of 14% at a molar ratio of 4:1 to β-CD; adjust the pH value of the system to 12 with 0.5M NaOH solution, and react in the dark for 8 h under ice bath conditions, then add absolute ethanol The reaction was terminated; finally, the mixture was dialyzed for 5 days and freeze-dried to obtain β-CD-COOH;
[0033] (2) Preparation of CD-PEG monomer: Prepare β-CD-COOH aqueous solution with a mass concentration of 0.5%, mix β-CD-COOH, EDC and DMAP at a molar ratio of 1:0.5:0.8, and stir at room temperature 0.5 h; then add PEG with a molar ratio of 1.9:1 to β-CD-COOH and a relative molecular mass of 8000 Da, stir and react at room temperature for 24 h; finally dialyze the mixture for 5 days and freeze-dry to obtain CD-PEG mon...
Embodiment 3
[0037] (1) Carboxylation modification of β-CD: prepare an aqueous solution of β-CD with a mass concentration of 1.8%, and mix β-CD, NaBr, and TEMPO uniformly at a molar ratio of 1:4:2 under ice bath conditions; then Add a NaClO solution with an available chlorine content of 7% at a molar ratio of 4.5:1 to β-CD; adjust the pH value of the system to 9 with a 0.5M NaOH solution, and react in the dark for 7 h under ice bath conditions, then add absolute ethanol The reaction was terminated; finally, the mixture was dialyzed for 3 days and freeze-dried to obtain β-CD-COOH;
[0038] (2) Preparation of CD-PEG monomer: Prepare β-CD-COOH aqueous solution with a mass concentration of 0.8%, mix β-CD-COOH, EDC and DMAP at a molar ratio of 1:1.2:2.5, and stir at room temperature 2 h; then add PEG with a molar ratio of 1.9:1 to β-CD-COOH and a relative molecular mass of 1000 Da, and stir at room temperature for 20 h; finally, dialyze the mixture for 5 d and freeze-dry to obtain CD-PEG monom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com