The preparation method of fine dimethenamid
A technology of refining dimethenamid and xylene, which is applied in the field of herbicides, can solve the problems of large-scale industrial production, and achieve the effects of simple operation, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The invention provides a method for preparing refined dimethenamid, the method comprising the following steps,
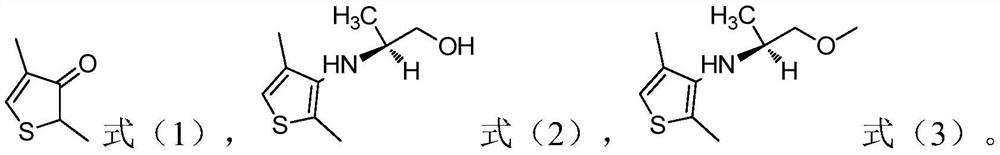
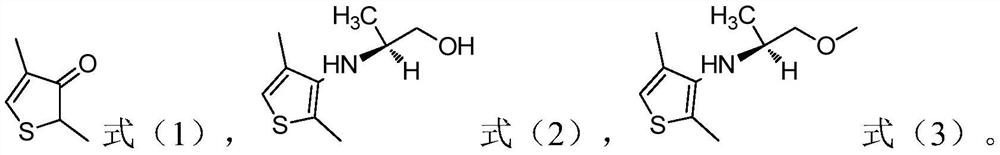
[0032] 1) In the presence of an acid, the compound of the structure shown in formula (1) is subjected to a first contact reaction with (2S)-1-hydroxypropyl-2-amine to obtain the compound of the structure shown in formula (2);
[0033] 2) In the presence of the first base, the compound of the structure shown in formula (2) is subjected to a second contact reaction with a methyl etherification reagent to obtain the compound of the structure shown in formula (3);
[0034] 3) In the presence of the second base, the compound of the structure shown in formula (3) is subjected to the third contact reaction with chloroacetyl chloride to obtain refined dimethenamid,
[0035]
[0036] According to the present invention, the structure of the refined dimethenamid is shown in formula (4),
[0037]
[0038] According to the present invention, the first contact react...
Embodiment 1
[0065] 1) Preparation of (S)-2-[(2,4-dimethyl-3-thienyl)amino]-L-propanol
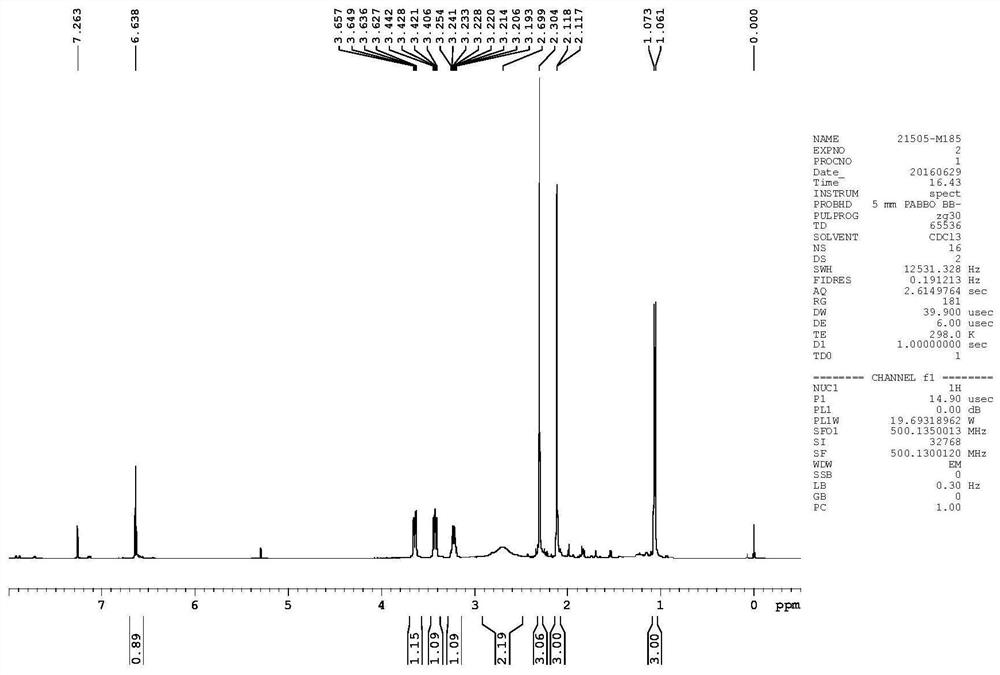
[0066] Add 10.4g content and be 98.5% by weight of 2,4-dimethyl-2,3-dihydrothiophen-3-one, 9.1g content of 99% by weight of L-alaninol ((2S) -1-hydroxypropyl-2-amine, ee value 99%) and 0.3g p-toluenesulfonic acid monohydrate, heated to boiling reaction (about 170°C). During the reaction, low boiling point substances (water) were distilled off. After 7 hours of reaction, the gas phase detection of 2,4-dimethyl-2,3-dihydrothiophen-3-one was less than 4%. Negative pressure removes the remaining L-alaninol (the internal temperature is up to 180°C, the top temperature is not more than 110°C, -96KPa, steaming until the top temperature drops indicates that the L-alaninol is exhausted). The residual viscous liquid in the remaining kettle was washed out with 50mL of dichloromethane, and then washed with 20ml of water. After layering, the solvent was removed to obtain 13.8g of the structural compound (dark visc...
Embodiment 2
[0082] 1) Preparation of (S)-2-[(2,4-dimethyl-3-thienyl)amino]-L-propanol
[0083] In the reaction flask, the content of adding 10.4g is 98.5% by weight of 2,4-dimethyl-2,3-dihydrothiophene-3-one, the content of 10g is 99% by weight of L-alaninol ((2S )-1-hydroxypropyl-2-amine, ee value 99%) and 0.8g p-toluenesulfonic acid, heated to boiling reaction (around 170°C). During the reaction, low boiling point substances (water) were distilled off. After 7 hours of reaction, the gas phase detection of 2,4-dimethyl-2,3-dihydrothiophen-3-one was less than 4%. Negative pressure removes the remaining L-alaninol (the internal temperature is up to 180°C, the top temperature is not more than 110°C, -96KPa, steaming until the top temperature drops indicates that the L-alaninol is exhausted). The residual viscous liquid in the remaining kettle was washed out with 50mL of dichloromethane, then washed with 20ml of water, and the solvent was removed after layering to obtain 13.6g of the struct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com