A kind of deuterotropane derivative and application thereof
A technology of tropane and its derivatives, applied in the chemical field, can solve the problems of complex operation, unfavorable animal basic research, and no advantage in popularization and application, and achieve the effects of slow metabolic degradation, good stability in the body, and high application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0049] Embodiment 1-1: A kind of deuterated tropane derivative
[0050] The present embodiment provides a kind of deuterotropane derivative FECNT-d 4 , the deuterotropane derivative FECNT-d 4 has the following structure:
[0051]
Embodiment 1-2
[0052] Embodiment 1-2: A kind of method for preparing deuterated tropine derivatives
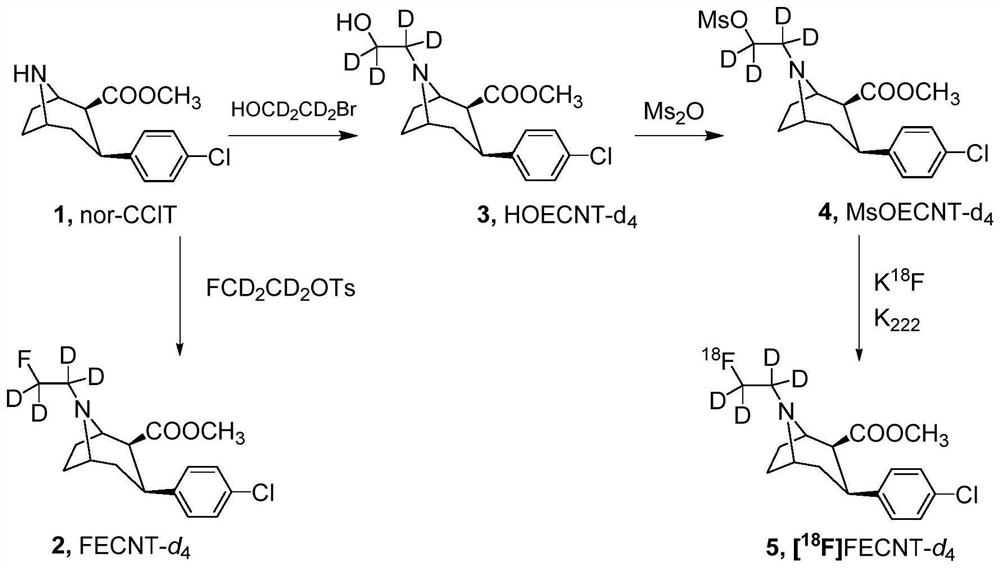
[0053] This embodiment provides the deuterated tropane derivative FECNT-d described in embodiment 1-1 4 The preparation method, described method is (deuterotropane derivative FECNT-d 4 For the synthetic route see figure 1 ):
[0054] Under nitrogen protection, 279mg nor-CClT (1mmol) and 222mg FCD 2 cd 2 OTs (1mmol) was dissolved in 8mL of methanol to obtain a mixed solution; 258mg N,N-diisopropylethylamine (DIPEA, 2mmol) was added to the mixed solution, and heated under reflux at 100°C for 24h to obtain a reflux solution; After the liquid was cooled to room temperature (25°C), 5mL NaOH solution (1mol) was added to the reflux liquid, stirred for 1h to react to obtain a reaction liquid; 5mL benzene was added to the reaction liquid, and concentrated under reduced pressure at 60°C to obtain a crude product ; The crude product is purified through silica gel chromatography to obtain deuterotr...
Embodiment 2-1
[0058] Embodiment 2-1: A kind of deuterated tropane derivative
[0059] This embodiment provides a kind of deuterotropane derivative [ 18 F] FECNT-d 4 , the deuterotropane derivatives [ 18 F] FECNT-d 4 has the following structure:
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com