Beta-carboboline GSK3beta/DYRK1A dual inhibitor and preparation method thereof and application of beta-carboboline GSK3beta/DYRK1A dual inhibitor in resisting Alzheimer's disease
A carboline, general formula technology, applied in the field of organic compound synthesis and medical application, can solve problems such as affecting axonal transport function, reducing acetylcholine content, affecting cognitive function and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
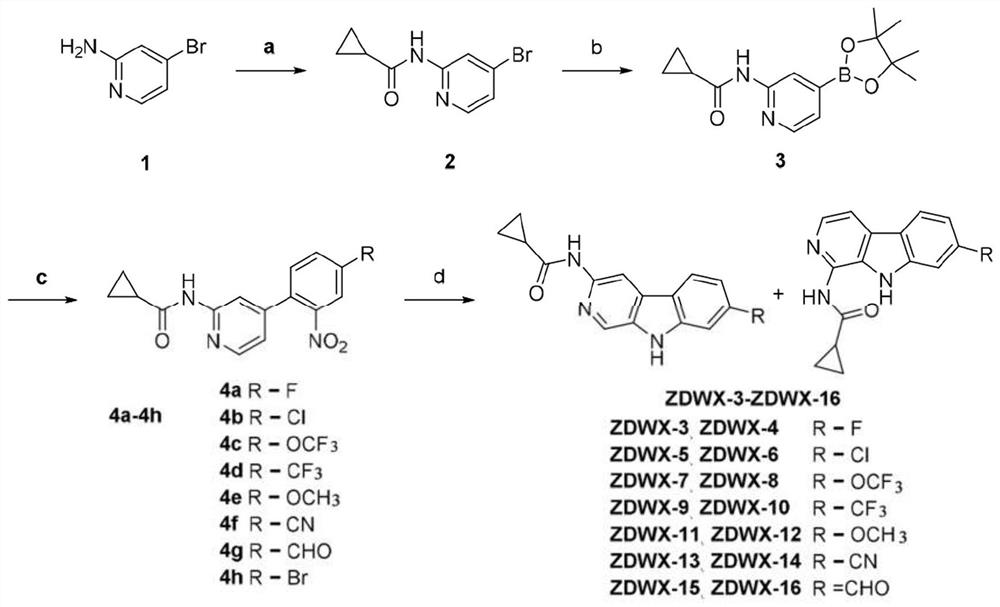
[0068] N-(4-Bromopyridin-2-yl)cyclopropanecarboxamide (Compound 2)
[0069]
[0070] Compound 4-bromo-2-aminopyridine (5g, 28.90mmol) and pyridine (3.43g, 43.35mmol) were dissolved in THF (50ml), and cyclopropanecarbonyl chloride (3.63g, 34.68mmol) was slowly added dropwise under ice-cooling After reacting for 4 hours, the reaction liquid was evaporated to dryness, then ice water was added thereto, and filtered by suction to obtain compound 2 as a white solid with a yield of 90%.
Embodiment 2
[0072] N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)cyclopropanecarboxamide (Compound 3)
[0073]
[0074] Compound 2 (3g, 12.44mmol) and bipinacol borate (3.79g, 14.93mmol) were dissolved in anhydrous dioxane (50ml), and KOAc (3.66g, 37.33mmol) and Pd(dppf ) Cl 2 , N 2 Under protection, after reacting at 90° C. for 12 h, the reaction solution was concentrated, water was added thereto and filtered with suction, and the filter cake was washed with petroleum ether and acetonitrile in sequence to obtain compound 3 as an off-white solid with a yield of 80%.
Embodiment 3
[0076] N-(4-(4-fluoro-2-nitrophenyl)pyridin-2-yl)cyclopropanecarboxamide (Compound 4a)
[0077]
[0078] Dissolve compound 3 (0.2g, 0.9mmol) and 2-bromo-5-fluoronitrobenzene (0.31g, 1.09mmol) in dioxane / water (8ml:2ml), add CS 2 CO 3 (0.37g, 2.73mmol) and Pd(dppf)Cl 2 , N 2 Under protection, after reacting at 95°C for 10 h, the reaction liquid was concentrated, ethyl acetate was added, extracted, and the concentrated intermediate obtained was purified by silica gel column to obtain the pure compound 4a as a light yellow solid with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com