Detection method of angiogenic peptide
An angiogenesis and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the detection method of angiogenesis inhibitors has not yet been established
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides an ultra-efficient liquid chromatographic analysis method of angiogenesis FPAT, which is as follows:
[0046] Test Instruments and Chromatography Conditions:
[0047] Waters H-CLASS Ultra High Performance Liquid Chromatography, Columns Bile silica gel as filler (2.1 × 100 mm, 1.7 μm, ACQUITY UPLC peptide beh, mixed with acetonitrile in 0.01 mol / L heptane sulfonate (pH value to 2.50) and acetonitrile to flow phase A, with acetonitrile to flow phase B, flow rate of 0.35 ml / min, the detection wavelength is 210 nm, the temperature is 60 ° C, the injection concentration is 1 mg / ml, the amount of injection is 0.8 ul, and the gradient elution is performed according to Table 1.
[0048] Table 1 Elution gradient
[0049]
[0050] Systematic Suitable Solution: Take LGX3, dissolve with water, and produce an LGX3 solution having a concentration of 0.1 mg / ml; Take the FPAT raw material, dissolved with LGX3 solution, and a systematic solution of 1 mg / ...
Embodiment 2
[0053] This embodiment provides an ultra-efficient liquid chromatographic analysis method of angiogenesis FPAT, which is as follows:
[0054] Test Instruments and Chromatography Conditions:
[0055] Waters H-Class Ultra Eco Liquid Chromatography, C18 Columns (2.1 × 100mm, 1.7 μm, ACQUITY UPLC PEPTIDE BEH, at 0.01 mol / L heptane sulfonate (pH value to 2.50) is a flow phase A, with acetonitrile to flow phase B, a flow rate of 0.35 ml / min, and the detection wavelength is 210 nm, temperature It was 60 ° C, the injection concentration was 1 mg / ml, and the amount of injection was 0.8 μL, and the gradient elution was followed.
[0056] Table 2 eluting gradient
[0057]
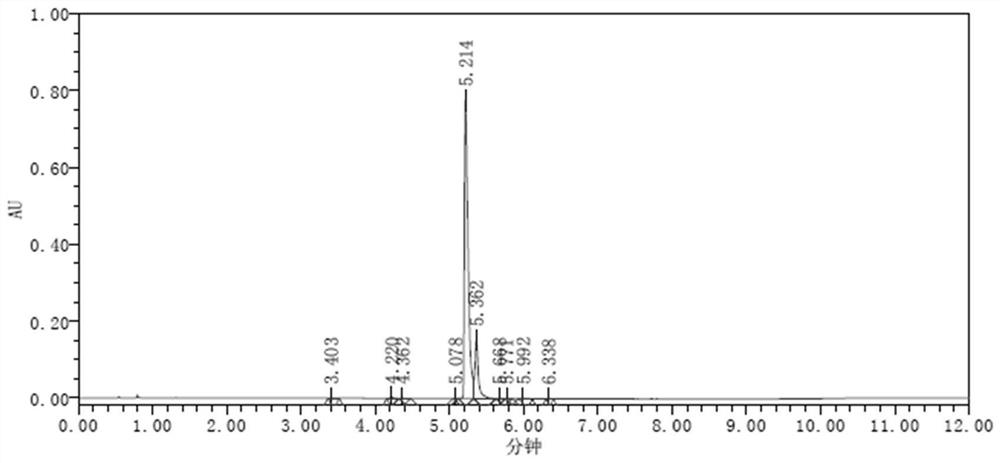
[0058] The FPAT raw material was dissolved in water into a sample solution of 1 mg / mL for injection. figure 2 As shown, it is seen from the figure that the main peak is quickly drawn, and there is no hanging column.
Embodiment 3
[0060] This embodiment provides an ultra-efficient liquid chromatographic analysis method of angiogenesis FPAT, which is as follows:
[0061] Test Instruments and Chromatography Conditions:
[0062] Waters H-Class Ultra Eco Liquid Chromatography, C18 Columns (2.1 × 100mm, 1.7 μm, ACQUITY UPLC PEPTIDE BEH, at 0.01 mol / L heptane sulfonate (pH value to 2.50) is a flow phase A, with acetonitrile to flow phase B, a flow rate of 0.35 ml / min, and the detection wavelength is 210 nm, temperature It was 60 ° C, the injection concentration was 1 mg / ml, and the amount of injection was 0.8 μL, and the gradient elution was followed.
[0063] Table 3 eluting gradient
[0064]
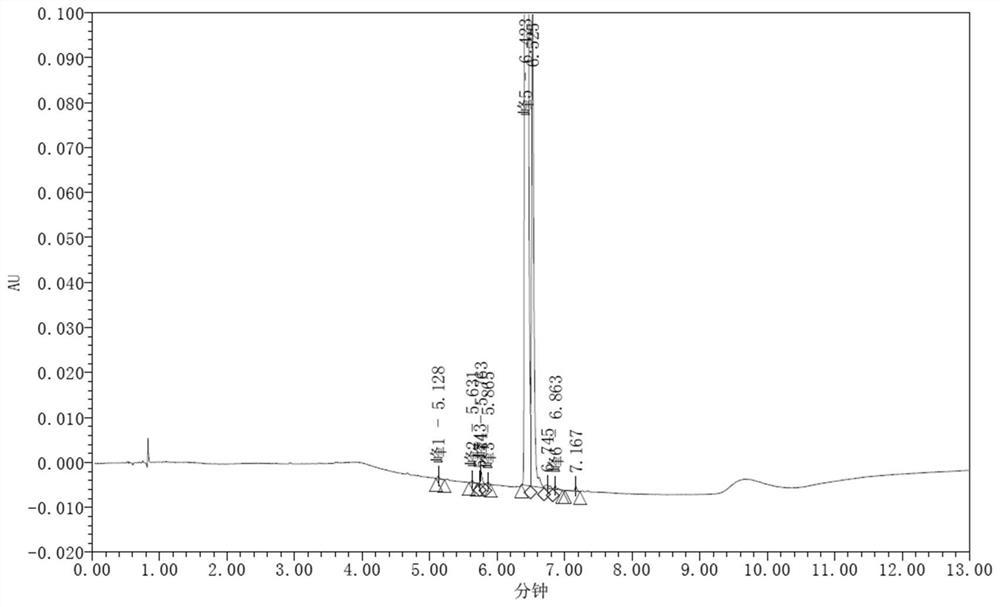
[0065] Determination of systematic solutions, image 3 As shown, the number of theoretical boards is calculated as 146510 in the FPAT peak; adjacent to the main peaks of post-surrogate LGX3 and the FPAT peak separation 1.60. However, the main peak of the raw material contains impurities and is severely trailing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specification | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com