Improved process of aldehyde and amine accelerant DHP
A technology of accelerators and aldehydes and amines, which is applied in the field of improved technology of aldehydes and amines accelerator DHP, can solve the problems of limited practical application, complex and unpleasant smell, etc., and achieve high market application value, light appearance color, and reduce residue effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
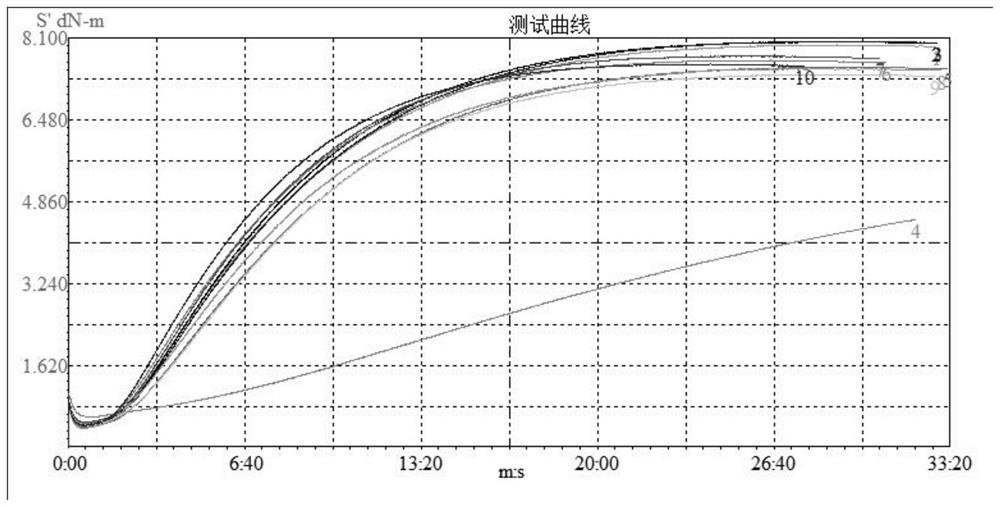
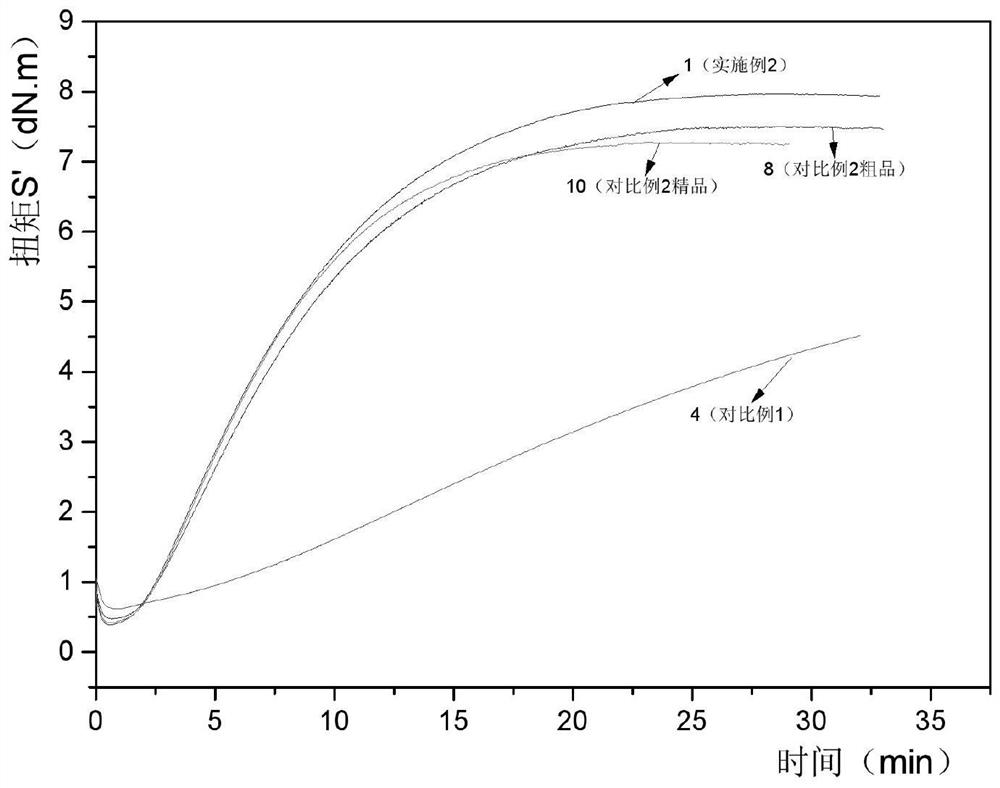
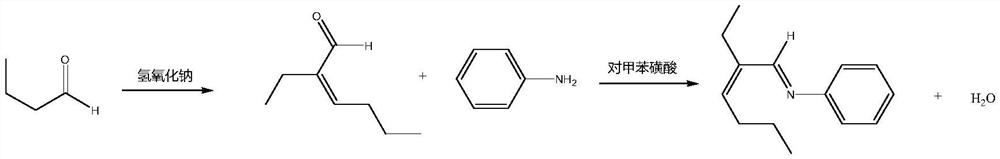
Image
Examples
Embodiment 1
[0039] Add 58.86g of n-heptanal and 1.40g of acetic acid into a 200ml flask, stir for 1h, cool down to 0°C, control the temperature at 0-5°C, slowly add 9.31g of aniline dropwise, and keep warm for 10h after the dropwise addition. Turn on the oil pump to keep the system under negative pressure, start to heat up, and distill off the water at 30-60°C; continue to heat up to 110-120°C to recover n-heptanal, and cool down to obtain 51.13g of the product, which is yellow and clear liquid, and the heptanal group is detected by gas chromatography DHP content is 60.52%, and heptanal and aniline residues are not detected.
Embodiment 2
[0041] Add 58.86g of n-heptanal and 1.40g of acetic acid into a 200ml flask, stir the reaction for 1h, control the temperature at 20-30°C, slowly add 9.31g of aniline dropwise, and continue to keep warm for 5h after the dropwise addition. Turn on the oil pump to keep the system under negative pressure, start to heat up, and distill off the water at 30-60°C; continue to heat up to 110-120°C to recover n-heptanal, and cool down to obtain 51.03g of the product, which is yellow and clear liquid, and the heptanal group is detected by gas chromatography The content of DHP is 63.21%, and no residues of heptanal and aniline are detected.
Embodiment 3
[0043] Add 58.86g of n-heptanal and 1.40g of acetic acid into a 200ml flask, stir the reaction for 1h, control the temperature at 30-40°C, slowly add 9.31g of aniline dropwise, and continue to keep warm for 4h after the dropwise addition. Turn on the oil pump to keep the system under negative pressure, start to heat up, and distill off the water at 30-60°C; continue to heat up to 110-120°C to recover n-heptanal, and cool down to obtain 51.06g of the product, which is yellow and clear liquid, and the heptanal group is detected by gas chromatography The content of DHP is 63.13%, and no residues of heptanal and aniline are detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com