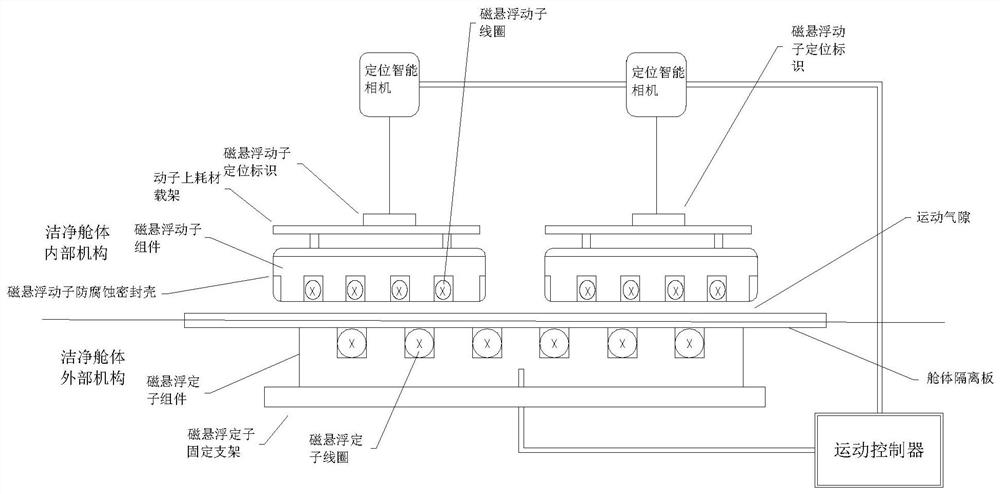
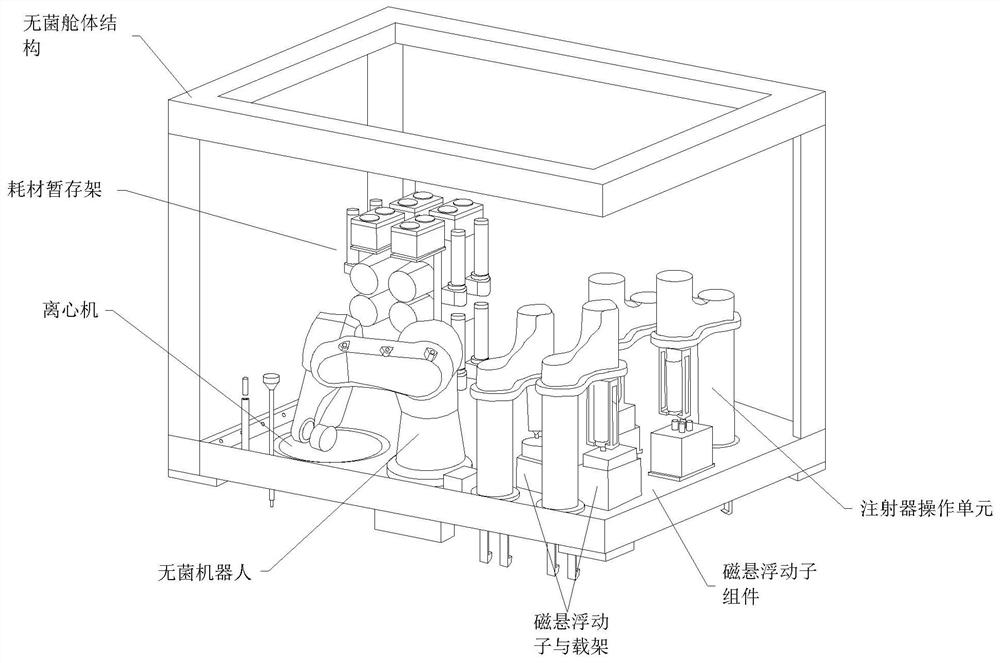
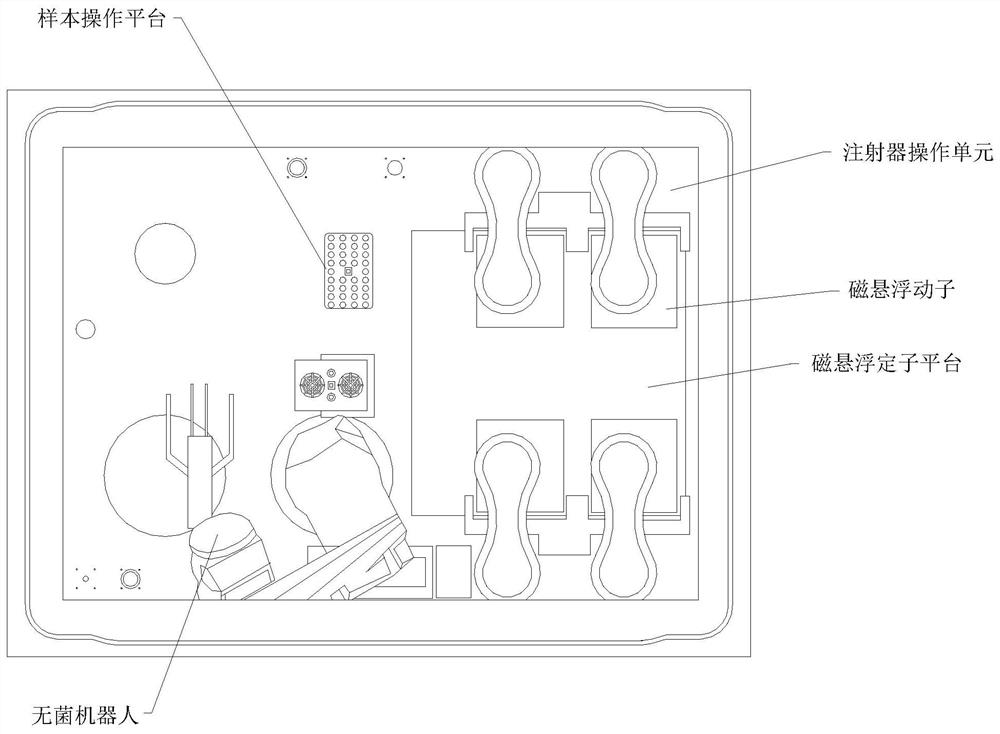
High-cleanliness biopharmaceutical sterile liquid operation platform based on magnetic suspension technology
A high-cleanliness, biopharmaceutical technology, used in workbenches, manufacturing tools, clean rooms, etc., can solve the problems of unfavorable effective contact and deep sterilization of gasification sterilants, unfavorable formation and maintenance of key laminar flow patterns, The complex spatial structure of the mechanical structure can reduce the risk of particle and microbial sedimentation, avoid the need for regular inspection and maintenance work, and improve the operability of personnel.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0067] Step 1: first sample sampling;
[0068] 1) The sterile robot removes the luer head cap from the sample culture bottle and transfers it to the magnetic levitation mover carrier specified by the software;
[0069] 2) The sterile robot removes the luer head cap from the sampling tube and transfers it to the magnetic levitation mover carrier specified by the software;
[0070] 3) The sterile robot removes the Luer head cap from the small-capacity syringe, and installs it into the gripper of the syringe operation unit specified by the software;
[0071] 4) The mover carrier carrying the culture bottle moves to the lower end of the syringe for precise positioning, cancels the suspended state, and locks the position;
[0072] 5) The magnetic levitation mover carrier is linked with the syringe operation unit, and the culture bottle is connected with the syringe;
[0073] 6) The syringe operation unit operates the syringe to draw the sample liquid with a specified volume;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com