Purification method for erythromycin thiocyanate
A technology of erythromycin thiocyanate and a purification method, which is applied in the field of biopharmaceuticals to achieve the effects of improved fermentation level, strong process adaptability, and quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
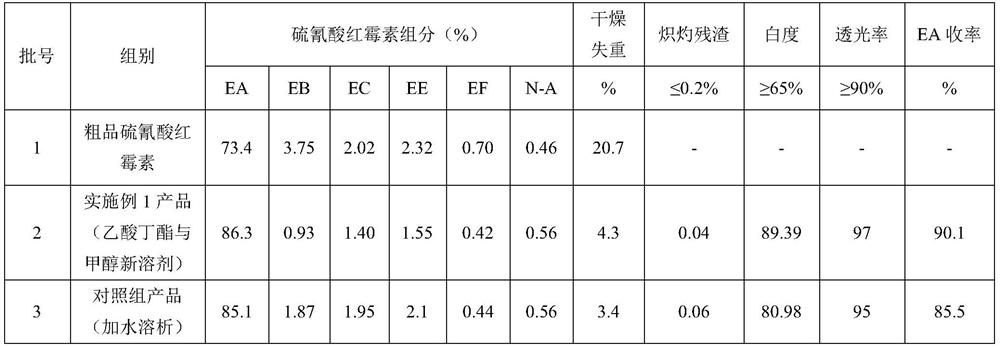
Embodiment 1
[0031] A purification method of erythromycin thiocyanate, said method comprising the following steps:
[0032] 1.1 Crude erythromycin thiocyanate was dissolved and phase-separated
[0033] Accurately weigh 500g of crude product erythromycin thiocyanate, measure 1650ml of butyl acetate by material ratio crude product erythromycin thiocyanate (g): butyl acetate (ml)=1:3.3, transfer butyl acetate to glass After the beaker (specification 3000ml), start stirring, add crude product erythromycin thiocyanate, heat up after adding, when the temperature of the system rises to 45°C, start to add NaOH solution with a mass concentration of 5%, and control the pH of the system to 10.4 until complete Dissolve, keep warm and stand still, separate the phases, and collect the upper layer of butyl acetate phase material.
[0034] 1.2 Add crystallization aids to crystallize
[0035] Preparation of crystallization aid: 40 g of solid NaSCN (1.25 mol equivalent) was added to 330 ml of H 2 In O (2...
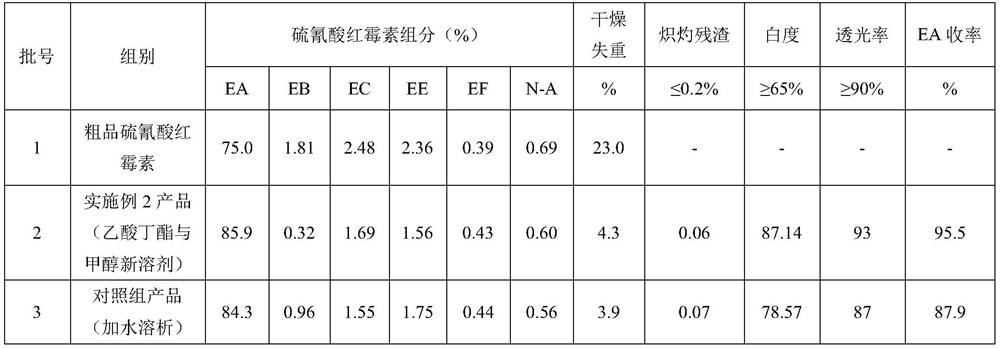
Embodiment 2
[0044] A purification method of erythromycin thiocyanate, said method comprising the following steps:
[0045] 2.1 Crude erythromycin thiocyanate dissolved and phase separated
[0046] Accurately weigh crude product erythromycin thiocyanate 2000g, measure butyl acetate 6600ml by material ratio crude product erythromycin thiocyanate (g): butyl acetate (ml)=1:3.3, transfer butyl acetate to glass After the dissolving tank (10000ml in size), start stirring, add crude product erythromycin thiocyanate, heat up after adding, when the temperature of the system rises to 45°C, start to add NaOH solution with a mass concentration of 5%, and control the pH of the system to 11 until Dissolve completely, keep warm and stand still, separate the phases, and collect the upper butyl acetate phase material.
[0047] 2.2 Crystallization by feeding crystallization aids
[0048] Preparation of crystallization aids: 190 g of solid NaSCN (1.49 mol equivalent) was added to 1320 ml of H 2In O (20% b...
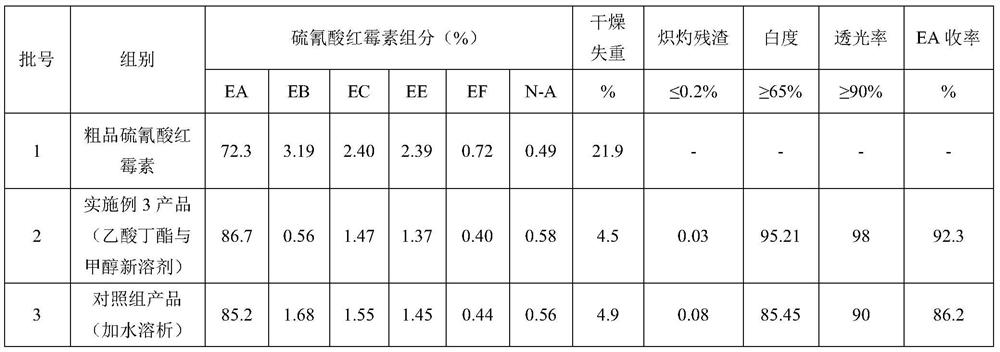
Embodiment 3
[0057] A purification method of erythromycin thiocyanate, said method comprising the following steps:
[0058] 3.1 Crude erythromycin thiocyanate dissolution and phase separation
[0059] Accurately weigh 36Kg of crude product erythromycin thiocyanate, measure 144L of butyl acetate by material ratio crude product erythromycin thiocyanate (g): butyl acetate (ml)=1:4, transfer butyl acetate to enamel After the glass dissolution tank (specification 250L), start stirring, add crude erythromycin thiocyanate, heat up after adding, when the system temperature rises to 40°C, start to add NaOH solution with a mass concentration of 10%, and control the pH of the system to 10 Until it is completely dissolved, keep it warm and stand still, separate the phases, and collect the upper layer of butyl acetate phase material.
[0060] 3.2 Crystallization by feeding crystallization aids
[0061] Preparation of crystallization aid: 2919g solid NaSCN (1.3mol equivalent) was added to 36LH 2 In O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com