Application of Y-39983 HCl in preparation of antiviral drugs
A technology of y-39983hcl and 1.y-39983hcl is applied in the application field of antiviral drugs to achieve the effect of broad-spectrum inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
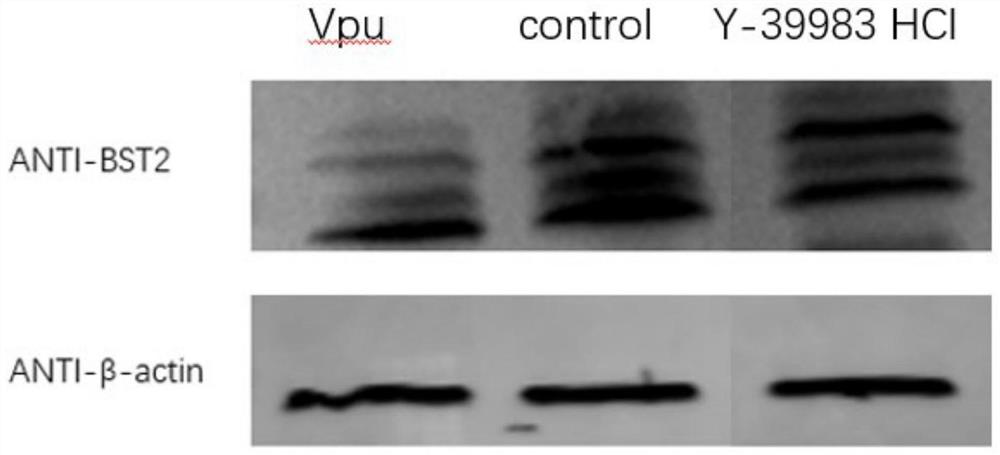
Image
Examples
Embodiment 1
[0025] 1.1 Cells and plasmids
[0026] HEK293T cells, TZM-bl cells, and Hela cells were all preserved by our laboratory. The NanobiT vector was purchased from Promega, and the pseudovirus packaging plasmids 11051 and 11035 were preserved by our laboratory.
[0027] 1.2 Main reagents
[0028] Y-39983 HCl used in in vitro experiments was purchased from Selleck Chemicals, with a purity of >99%.
[0029] 1.3 Y-39983 HCl Toxicity Test
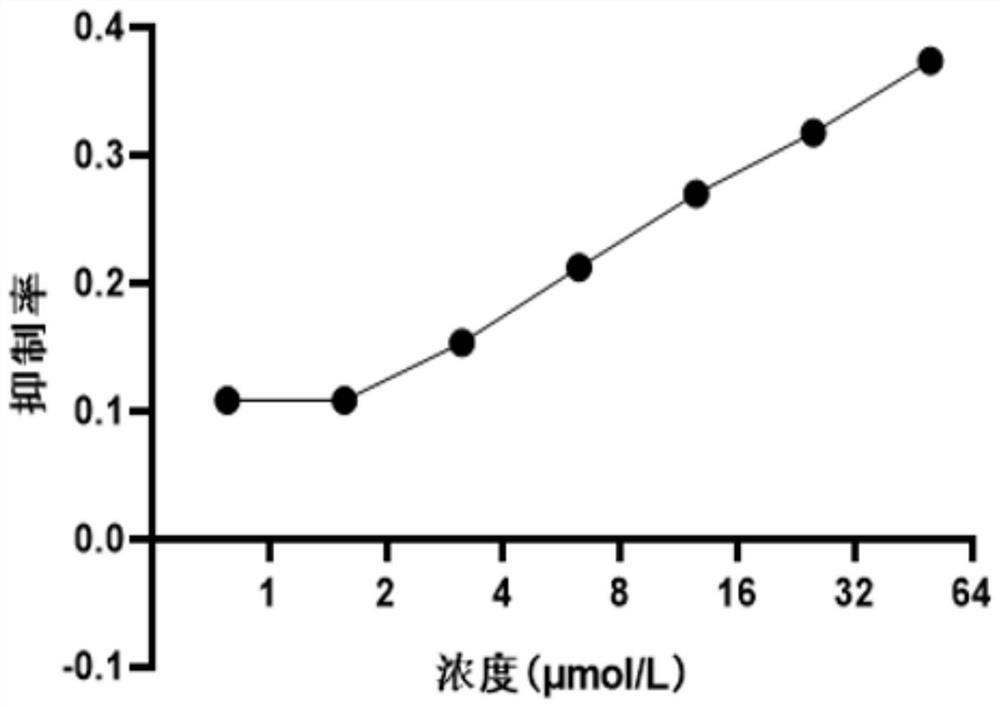
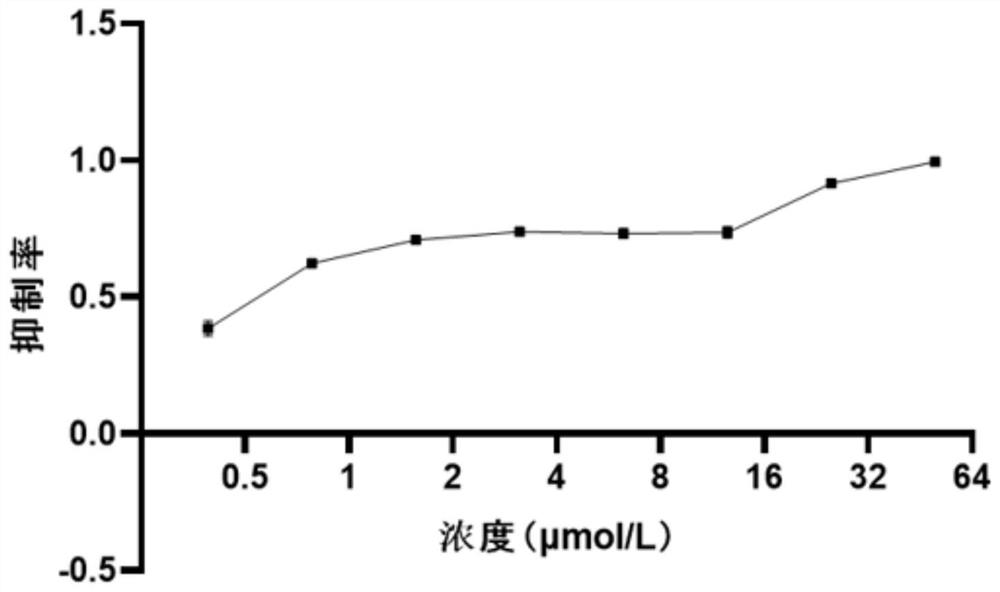
[0030] In order to detect the toxicity of compound Y-39983 HCl, compound Y-39983 HCl was added to HEK293T cells at a final concentration of 0 μmol / L-50 μmol / L. 48 hours after treatment, cell viability was detected using CCK-8. The relative cell viability was calculated as the percentage of DMSO-treated control cells. IC50 values of drugs were calculated using GraphPad Prism 8.0 software. Such as figure 1 It is shown that when the drug is calculated by the activity of the cells at 6.25 μM, the inhibition rate of the cell activity is 21.2%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com