Recombinant human XVII type collagen, and preparation method and application thereof
A collagen and application technology, applied in the field of genetic engineering, can solve the problem of cell adhesion active recombinant human type XVII collagen failing to be produced on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
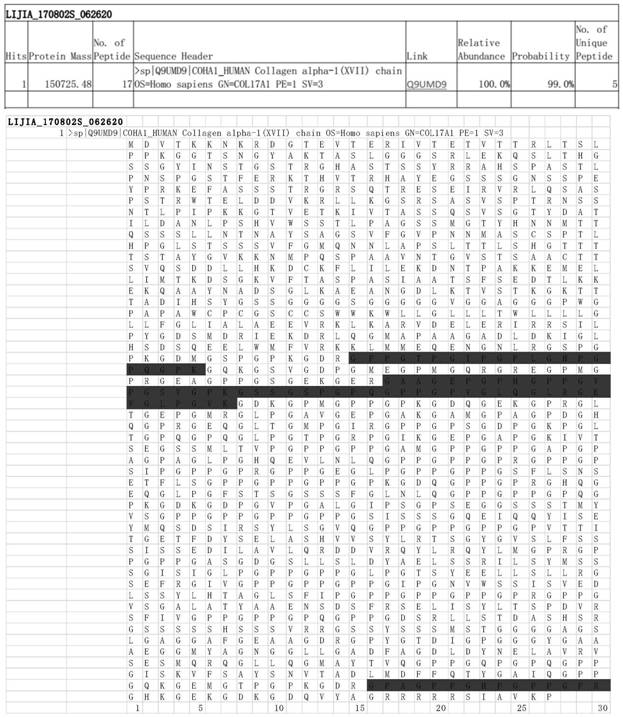
[0066] Example 1. Construction, expression and identification of recombinant human type XVII collagen
[0067] (1) Design of amino acid sequence of recombinant human type XVII collagen
[0068] In the present invention, based on the optimization of human type XVII collagen sequence, the specific sequence reference of human type XVII collagen is: Uniprot Q9UMD9-1 sequence (https: / / www.uniprot.org / uniprot / Q9UMD9) and NCBI reference sequence Q9UMD9. 3 (https: / / www.ncbi.nlm.nih.gov / protein / Q9UMD9.3), the two sequences are identical, as shown in SEQ ID NO.1.
[0069] SEQ ID NO.1:
[0070]
[0071]
[0072] The bold underlined part of the above sequence SEQ ID NO.1 is the sequence selected in the present invention, with a total of 233 amino acids. The sequence selected in the present invention is a combined sequence selected after optimization and screening from multiple helical region sequences such as the 15th helical region, the carboxyl terminal region, and the middle re...
Embodiment 2
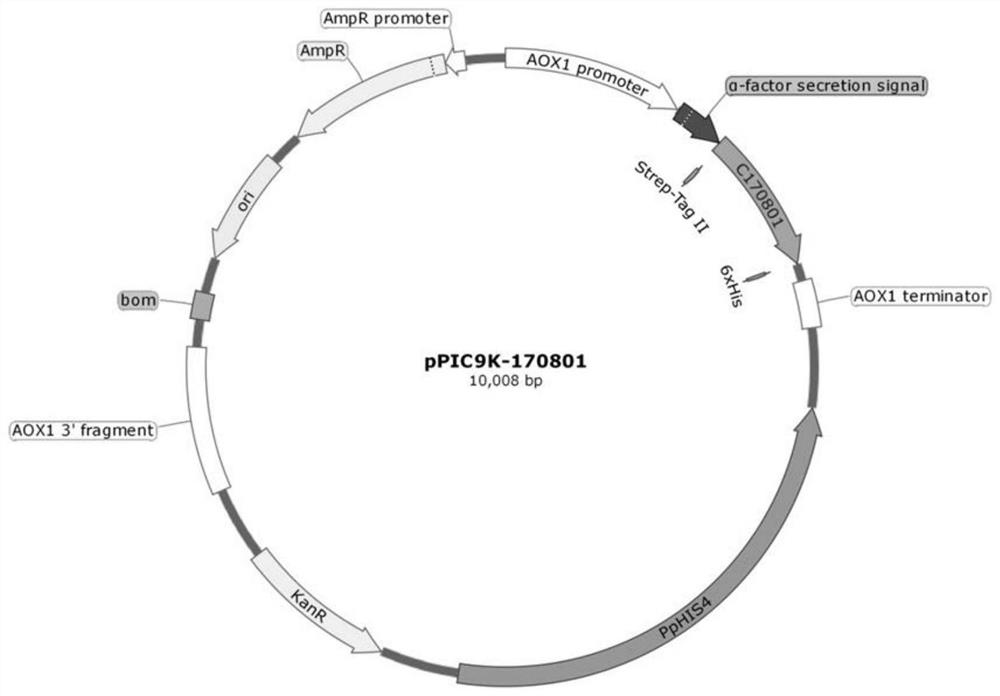
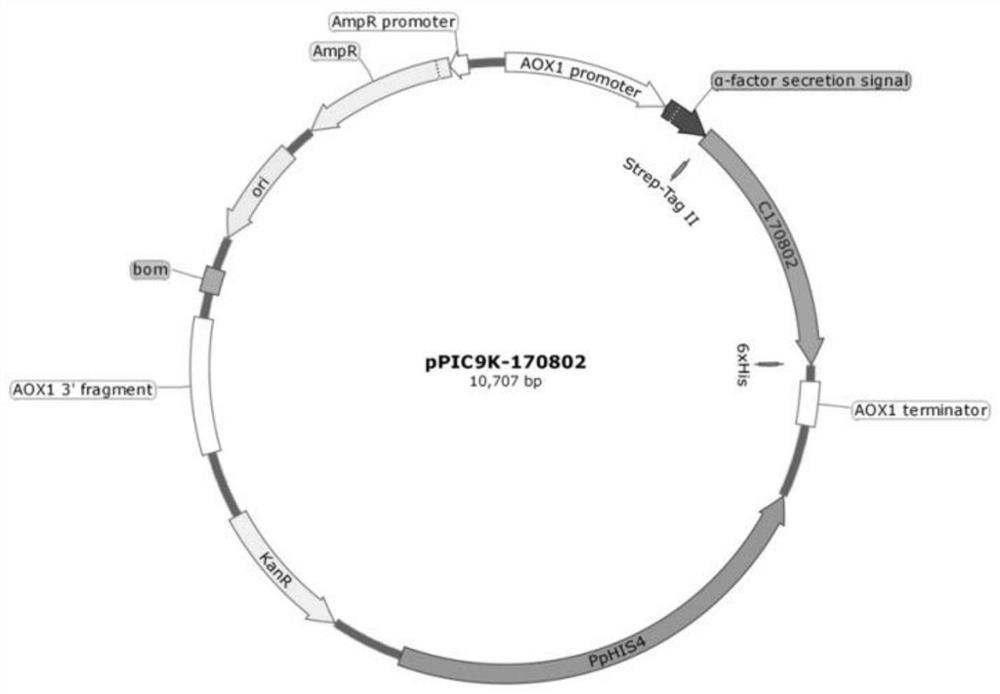
[0131] Example 2. Pilot-scale fermentation and protein purification of genetically engineered bacteria
[0132] (1) Pilot fermentation
[0133] The engineered bacteria containing pPIC9K-170801 and pPIC9K-170802 respectively were fermented in a 50L-500L linkage pilot test to obtain a fermentation broth containing recombinant human type XVII collagen to achieve large-scale expression of recombinant human type XVII collagen 170801 and 170802 Production.
[0134] Seed medium YPG (recipe: yeast powder 10g / L, yeast peptone FP102 20g / L, anhydrous glycerin 10g / L); fermentation medium (recipe: NH 4 h 2 PO 4 190.4g / L, KH 2 PO 4 10.06g / L, CaSO 4 2H 2 O 1.18g / L, K 2 SO 4 18.2g / L, MgSO 4 ·7H 2 O 14.9g / L, glycerol 40g / L); feed medium (50% W / V glycerol, add 12mL PTM per liter 1 trace elements); induction medium (100% methanol, 12 mL PTM per liter 1 trace elements); where PTM 1 Sterilize by filtration with a 0.22 μm membrane filter and store at 4°C. After the fermentation med...
Embodiment 3
[0155] Example 3. Cell adhesion activity detection of recombinant collagen
[0156] References for Cell Adhesion Detection Method of Recombinant Collagen Juming Yao, Satoshi Yanagisawa, Tetsuo Asakura. Design, Expression and Characterization of Collagen-Like Proteins Based on the Cell Adhesive and Crosslinking Sequences Derived from Native Collagens, J Biochem.136, 643-649 (2004) . Entrusted to the Functional Nanomaterials and Biomedical Testing Laboratory of Changzhou University School of Pharmacy to complete.
[0157] Specific implementation method:
[0158] NIH / 3T3 cells were cultured normally (purchased from the Cell Bank of the Chinese Academy of Sciences, Cat. No. GNM6, and the cultivation and passage methods were performed according to the instructions of the cells). Take 170801 and 170802 protein purification and freeze-dried products, and the reference substance is natural human collagen (purchased from Sigma, item number C7774) and bovine serum albumin (BSA, purchase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
| Apparent molecular weight | aaaaa | aaaaa |
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com