Preparation method of hapten acetyl iodothyroxine active coupling reagent
The technology of thyroxine and coupling reagent is applied in the field of preparation of hapten acetyliodothyronine active coupling reagent, which can solve the problems such as difficulty in obtaining raw materials, poor solubility, long reaction time, etc. The effect of reducing costs and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
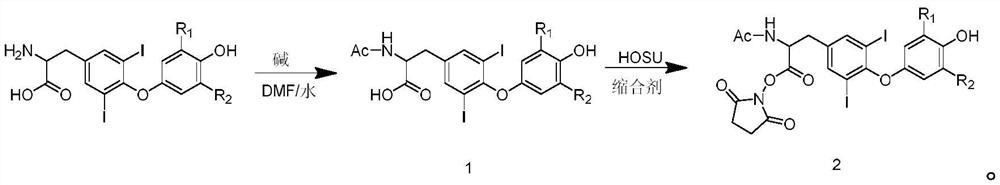
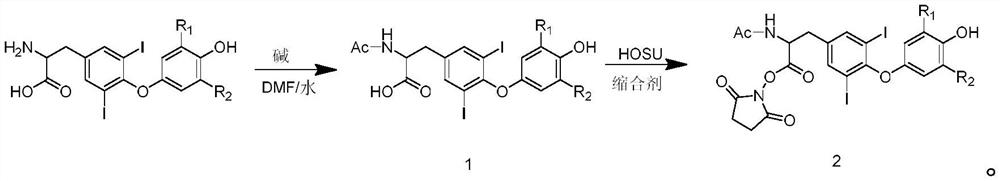
[0049] The invention discloses a preparation method of a hapten acetyliodothyronine active coupling reagent, which comprises the following steps:
[0050] S1, preparation of acetic acid active ester;
[0051] S2, reacting the prepared acetic acid active ester with iodothyroxine to synthesize intermediate compound 1;
[0052] S3, reacting the intermediate compound 1 with N-hydroxysuccinimide and a condensing agent to synthesize the hapten acetyliodothyronine active coupling reagent 2;
[0053] The synthetic reaction formula is:
[0054]
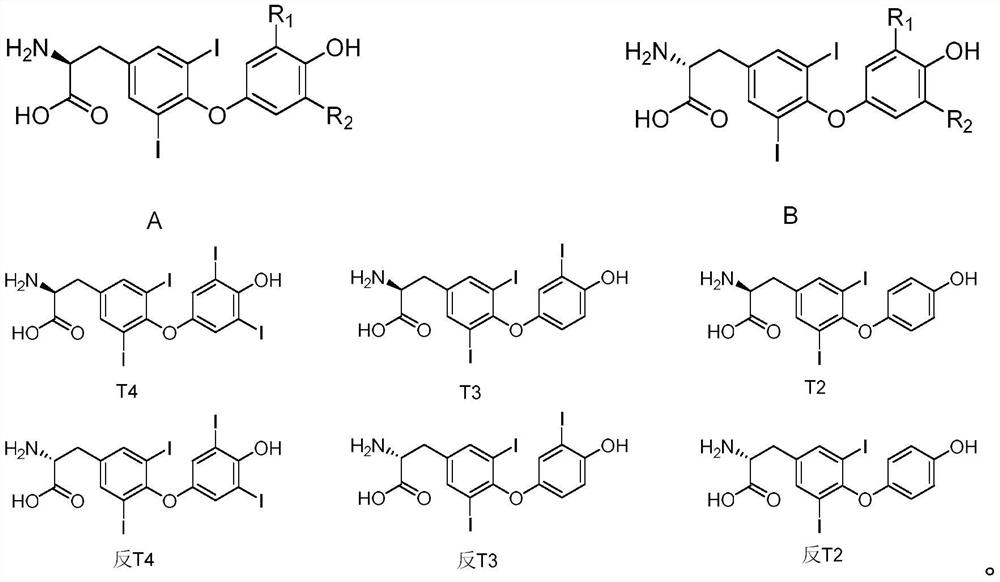
[0055] Wherein, the general formula of iodothyroxine is shown in formula A and formula B, and formula B is the trans form of formula A;
[0056] where the R 1 and R 2 The group is hydrogen or iodine; formula A includes T 4 , T 3 or T 2 ;Formula B includes anti-T 4 , Anti-T 3 and anti-T 2 ;
[0057] The specific chemical formula is as follows:
[0058]
[0059] Specifically, the specific steps for preparing acetic acid active es...
specific example 1
[0073]
[0074] The specific experimental conditions for the synthesis of intermediate compound 1 in S1 and S2 include: under nitrogen protection, acetic acid (0.7g, 3eq) and HOSU (1.3g, 3eq) were added to DMF (10ml), and EDC was slowly added below 20°C. HCl (2.2g, 3eq), react at 25°C for more than 1h. Then T4 (3g, 1eq) was added to DMF (15ml), and K 2 CO 3 (1.6g, 3eq) was added to water (20ml) to dissolve, then mixed to dissolve T4. Then add the reacted acetic acid active ester solution dropwise into the T4 solution, and react at room temperature for 1 hour. After the reaction is completed, adjust the pH to be acidic with dilute HCl aqueous solution, extract 3 times with an appropriate amount of EA, wash 2 times with saturated NaCl aqueous solution, and wash the EA solution with Anhydrous Na 2 SO 4 Dry, remove EA in a rotary evaporator, and use it directly for the next step without purification;
[0075] S3. Synthesis of product 2: Under nitrogen protection, intermedi...
specific example 2
[0077]
[0078] Synthesis of S1 and S2, intermediate compound 1: under nitrogen protection, acetic acid (0.82g, 3eq) and HOSU (1.6g, 3eq) were added to DMF (10ml), and EDC·HCl (2.65g , 3eq), react at 25°C for more than 1h. Then T3 (3g, 1eq) was added to DMF (15ml), K2CO3 (1.9g, 3eq) was added to water (20ml) to dissolve, and then mixed to dissolve T3. Then add the reacted acetic acid active ester solution dropwise into the T3 solution, and react at room temperature for more than 1 hour. After the reaction is completed, adjust the pH to be acidic with dilute HCl aqueous solution, extract 3 times with an appropriate amount of EA, wash 2 times with saturated NaCl aqueous solution, and EA solution with anhydrous Na 2 SO 4 Dry, remove EA in a rotary evaporator, and use it directly for the next step without purification;
[0079] S3. Synthesis of product 2: under nitrogen protection, compound 1 (1.5g, 1eq) and HOSU (0.3g, 1.2eq) were added to DMF (20ml), and EDC·HCl (0.5g, 1.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com