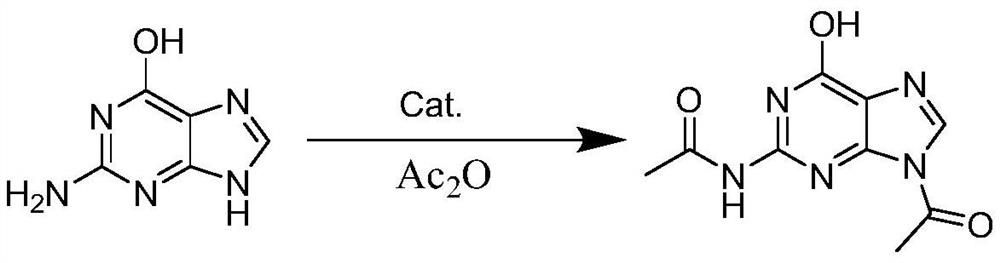
Synthesis method of 2-acetamido-9-acetylpurine
A technology of acetyl purine and acetyl amino, which is applied in the field of synthesis of 2-acetylamino-9-acetyl purine, which can solve the problems of three wastes, easy removal of acetyl groups, and troublesome treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthetic method of 2-acetylamino-9-acetylpurine
[0024] The preparation method of 2-acetylamino-9-acetylpurine provided in this example comprises the following steps:
[0025] Guanine (10g, 66.2mmol, 1eq), acetic anhydride (100g, 0.980mol) and tetrabutylammonium bromide (0.47g, 1.46mmol, 0.022eq) were added to the reaction vessel, the temperature was raised to 110°C, and the reaction was stirred 2 hours, then cooled to 25 ° C, filtered, the solid was separated from the filtrate, and the solid was dried at 50 ° C to obtain 15.2 g of 2-acetylamino-9-acetyl-6-hydroxypurine, the yield was 97.6%, the purity to 99.5%, the filtrate was concentrated under reduced pressure at 85°C, and the acetic acid could be recovered and used mechanically after separation.
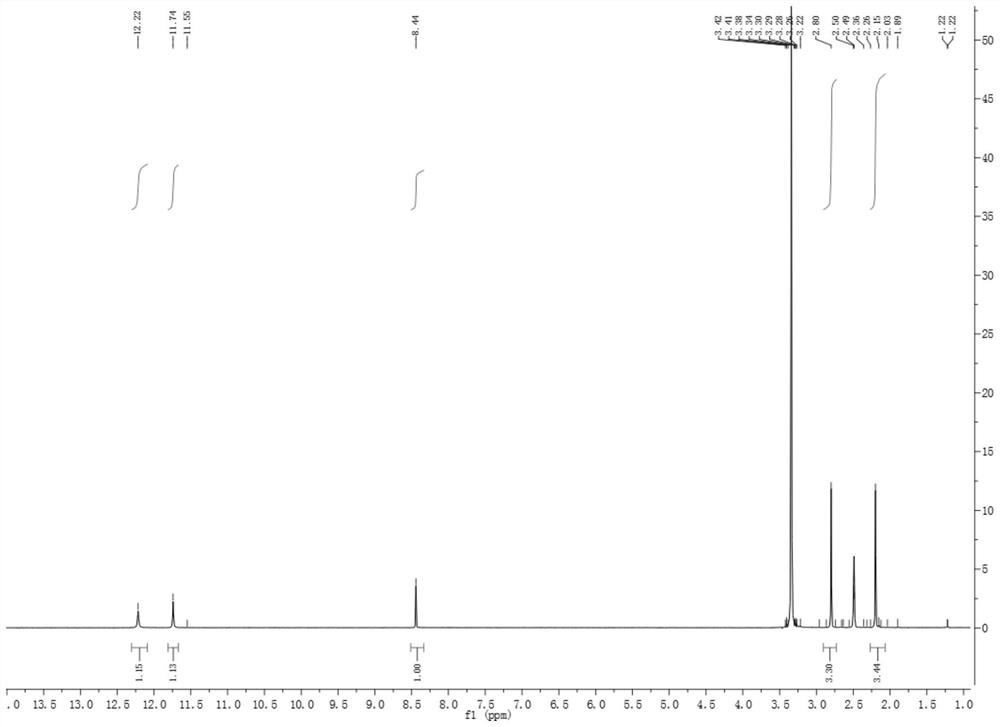
[0026] figure 1 It is the hydrogen spectrum of 2-acetylamino-9-acetylpurine in Example 1 of the present invention.
[0027] like figure 1 As shown, the hydrogen spectrum data of 2-acetylamino-9-acetylpurine are ...
Embodiment 2
[0030] Screening of Catalysts in the Synthesis of 2-Acetylamino-9-Acetylpurine
[0031] In this example, the acetylation catalyst in the preparation method of 2-acetylamino-9-acetylpurine was screened, and the specific experimental operation was the same as in Example 1, the only difference being that the acylation catalyst used was different or the amount or reaction time was long The specific results are shown in Table 1.
[0032] Table 1 Catalyst Screening Table
[0033] serial number catalyst equivalent Response time yield purity 1 none - 2h Trace - 2 Tetrabutylammonium chloride 0.022eq 2h 96.8% 99.5% 3 Tetrabutylammonium bromide 0.05eq 2h 98.9% 99.3% 4 4-Methylaminopyridine 0.022eq 2h 38.1% - 5 p-Toluenesulfonic acid 0.022eq 2h 22.3% - 6 Tetramethylammonium chloride 0.022eq 2h 87.5% 99.3% 7 Dicyclohexylcarbodiimide 0.022eq 2h 40.5% - 8 pyridine 0.022eq 2h 35.0% - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com