Method for detecting glycated serum albumin by using boric acid affinity principle
A serum albumin and glycosylation technology, applied in the field of glycosylated serum albumin detection, can solve the problems of poor stability of enzyme and antibody protein, complicated steps, time-consuming, etc., and achieve the effect of stable reagents and rapid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
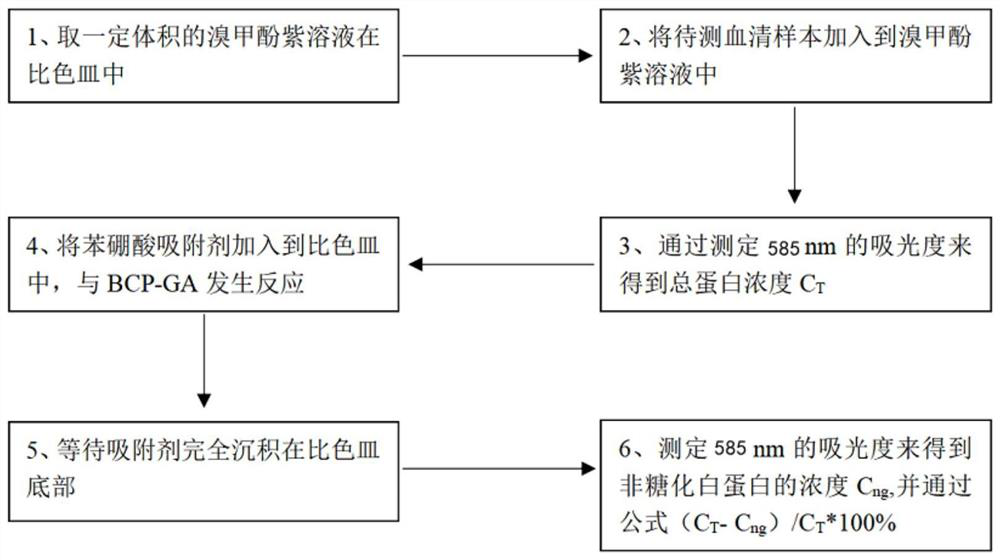
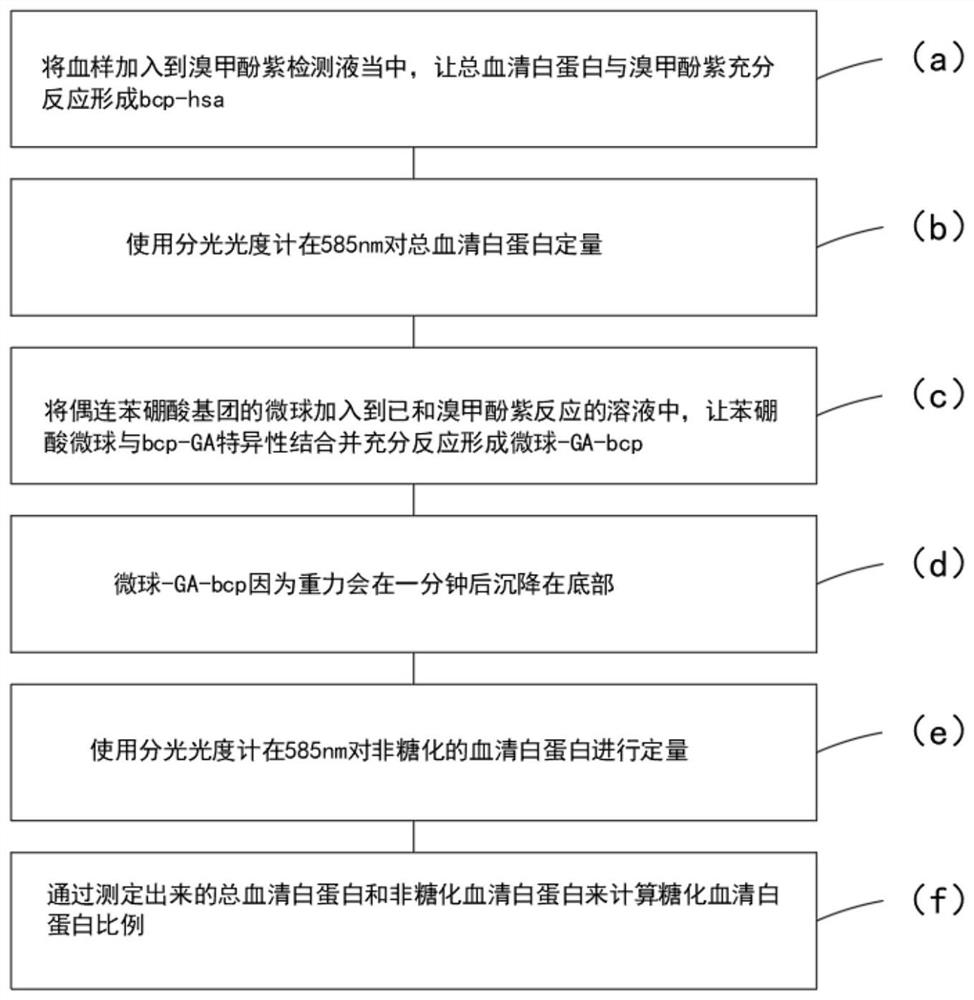
[0037] refer to Figure 1-2 , a method for detecting glycosylated serum albumin using the principle of boric acid affinity, characterized in that it comprises the following steps:
[0038] S1, get a certain volume of bromocresol violet solution in a cuvette;
[0039] S2, adding the serum sample to be tested into the bromocresol violet solution;
[0040] S3, obtain the total protein concentration C by measuring the absorbance at 585nm T ;
[0041] S4, adding the phenylboronic acid adsorbent to the cuvette to react with BCP-GA;
[0042] S5, waiting for the adsorbent to be completely deposited on the bottom of the cuvette;
[0043] S6, measure the absorbance at 585nm to obtain the concentration C of non-glycated albumin ng , and through the formula (C T -C ng ) / C T *100% is calculated as a percentage of serum albumin.
[0044] Wherein, steps S1, S2 and S3 are to obtain the absorbance A0 of the bromocresol violet solution at 585 nm under alkaline conditions, that is, the ...
Embodiment 2
[0061] The difference with Example 1 is:
[0062] The repetition rate of the method
[0063] Intra-batch repeatability test: Take the same serum sample and repeat the test 20 times continuously, and calculate the CV value. Batch-to-batch repeatability test: divide the same serum sample into 20 parts, store in the refrigerator, take 1 part for testing every day, for a total of 20 days, and calculate the CV value. The intra-assay CV value and the inter-assay (day) CV value should be less than 5% according to the requirements of the National Committee for Clinical Laboratory Standardization (NCCLS) documents, and the method meets the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com