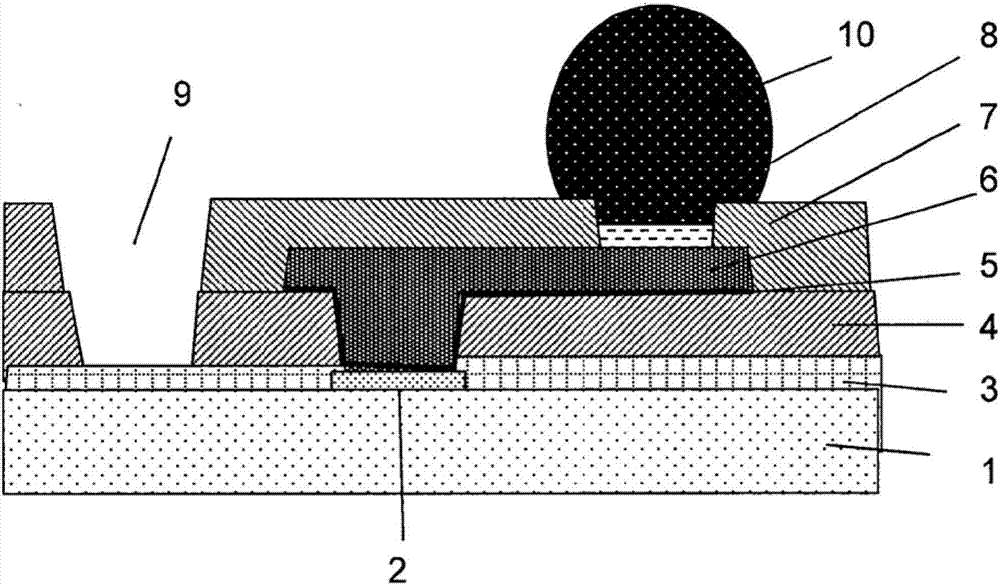
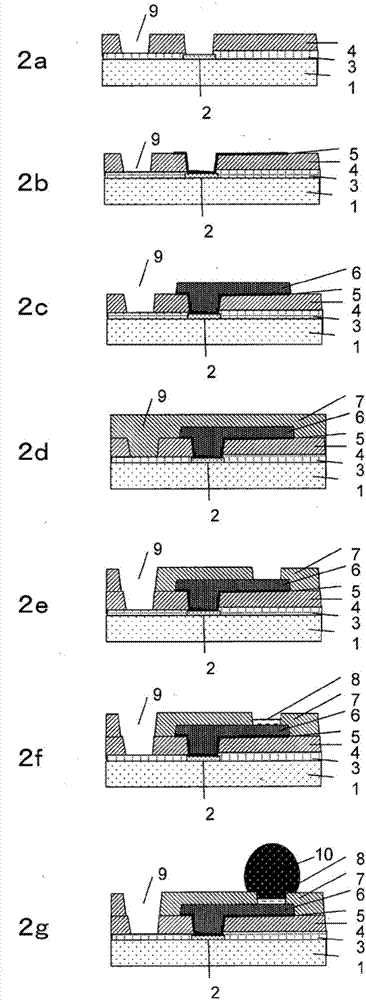
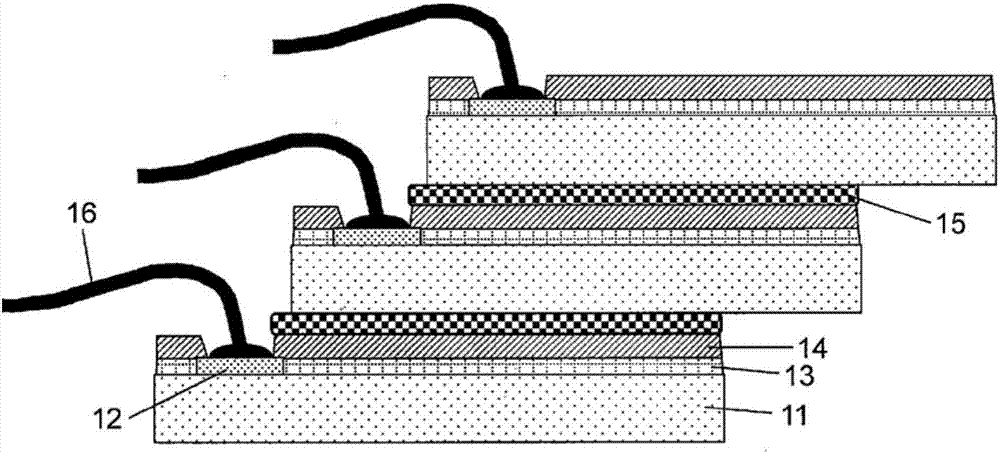
Resin, photosensitive resin composition, and electronic component and display device each using same
一种树脂、树脂膜的技术,应用在物,医药中间体的5,5’-二羟基-4,4’-二氨基联领域,能够解决选择性不高、收率低等问题,达到吸光度低、线性热膨胀系数小的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0284] Hereinafter, the implementation will be enumerated in the order of (1) resin, photosensitive resin composition, (2) 5,5'-dihydroxy-4,4'-diaminobiphenyl derivative, (3) dibromobiphenyl derivative The present invention is described by way of examples, but the present invention is not limited to these examples.
[0285]
[0286] The evaluation of the resin and resin composition in an Example was performed by the following method.
[0287] 1) Measuring method of film thickness
[0288] Using Lambda Ace STM-602 manufactured by Dainippon Screen Mfg. Co., Ltd., both the resin film and the coating film after heat-curing were measured at a refractive index of 1.629.
[0289] 2) Determination method of linear thermal expansion coefficient
[0290] The resin solution was spin-coated on an 8-inch silicon wafer, followed by baking for 3 minutes on a 120° C. hot plate (using a coating and developing device Act-8 manufactured by Tokyo Electron Limited.) to obtain a resin film.
...
Synthetic example 1
[0322] Synthesis example 1 Synthesis of hydroxyl-containing diamine compound (a)
[0323] Dissolve 17.6 g (0.05 mol) of 2,2'-bis(trifluoromethyl)-5,5'-dihydroxybenzidine in 100 mL of acetone and 17.4 g (0.3 mol) of propylene oxide, and cool to -15°C . A solution obtained by dissolving 20.4 g (0.11 mol) of 3-nitrobenzoyl chloride in 100 mL of acetone was dropped there. After completion of the dropping, the reaction was carried out at -15°C for 4 hours, and then returned to room temperature. The precipitated white solid was obtained by filtration, and vacuum-dried at 50°C.
[0324] 30 g of the obtained white solid was put into a 300 mL stainless steel autoclave, dispersed in 250 mL of methyl cellosolve, and 2 g of 5% palladium-carbon was added. Hydrogen gas is introduced into it with a balloon, and the reduction reaction is carried out at room temperature. After about 2 hours, the reaction was terminated after confirming that the balloon was no longer deflated. After comple...
Synthetic example 2
[0327] Synthesis example 2 Synthesis of hydroxyl-containing diamine compound (b)
[0328] Change 2,2'-bis(trifluoromethyl)-5,5'-dihydroxybenzidine 17.6 g (0.05 mol) to 2,2'-dimethyl-5,5'-dihydroxybenzidine 17.6 g (0.05 mol) and except that, it carried out similarly to the synthesis example 1, and obtained the hydroxyl group containing diamine compound (b) represented by the following formula. The resulting solid was used directly in the reaction.
[0329] [chemical formula 29]
[0330]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| residual stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com