Application of FG4592 in preparation of medicine for preventing and treating chronic kidney disease caused by ischemia-reperfusion injury
A technology for chronic kidney disease and ischemia-reperfusion, applied in drug combinations, cardiovascular system diseases, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
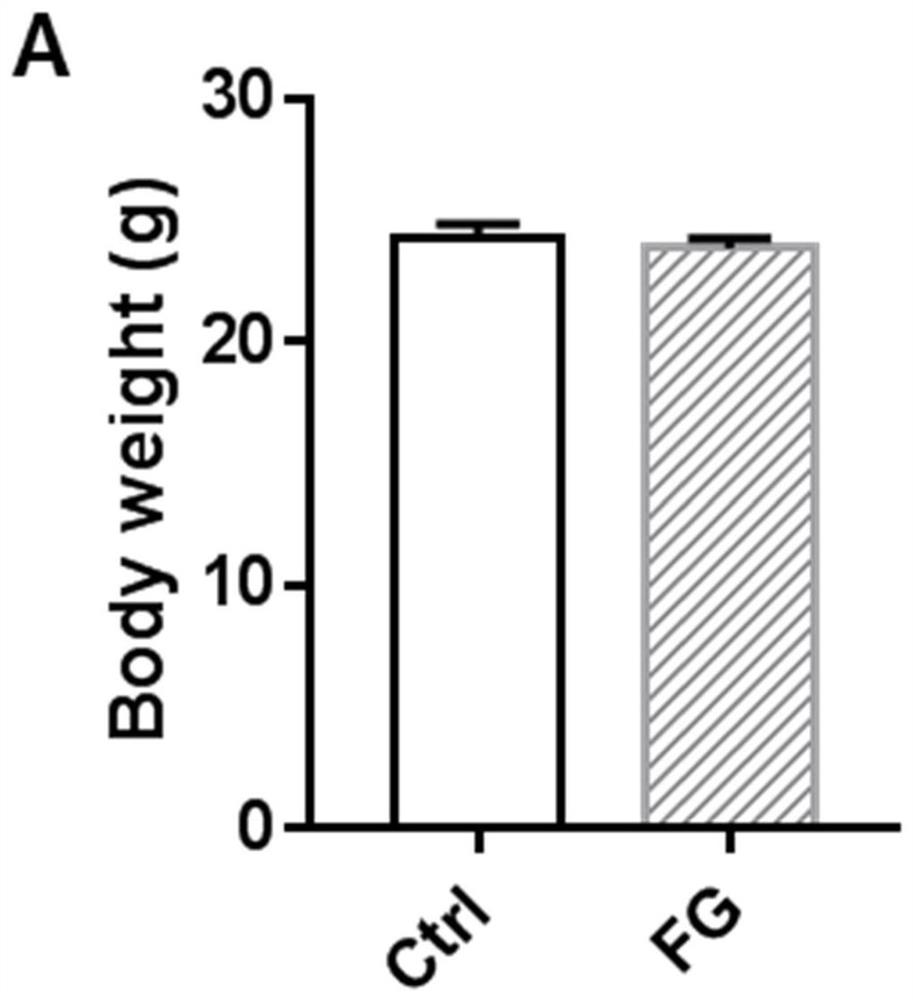
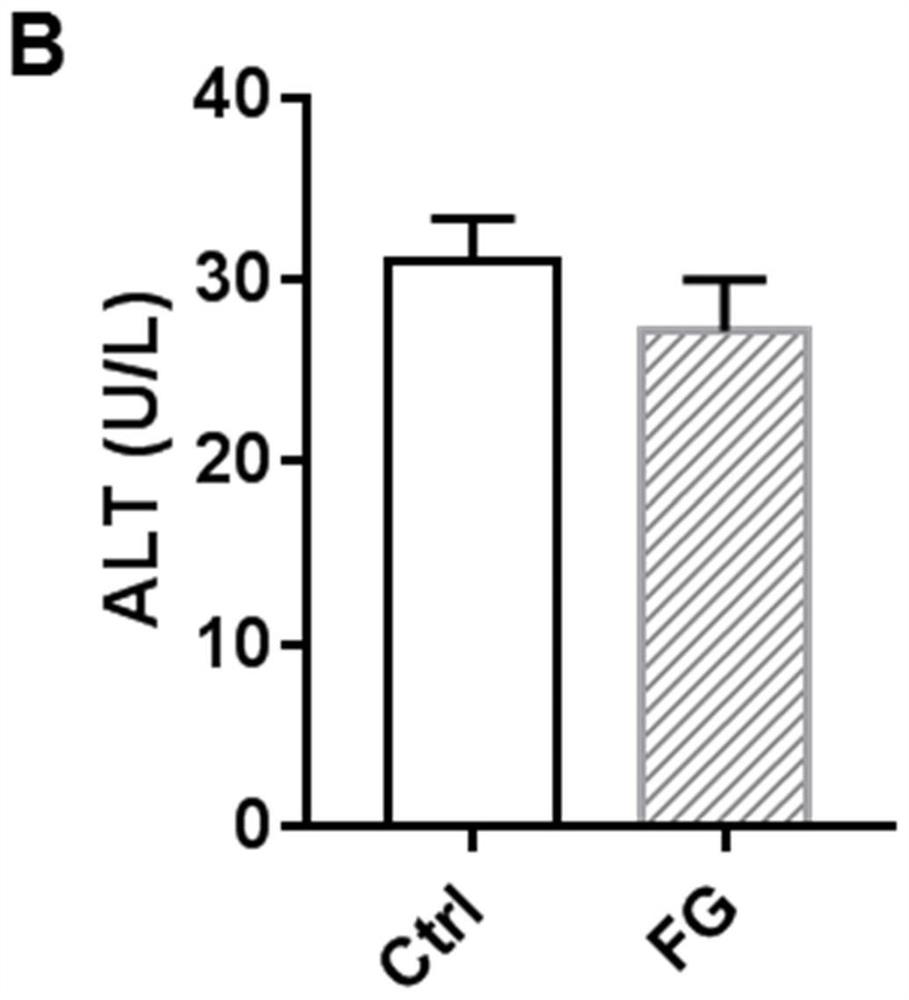
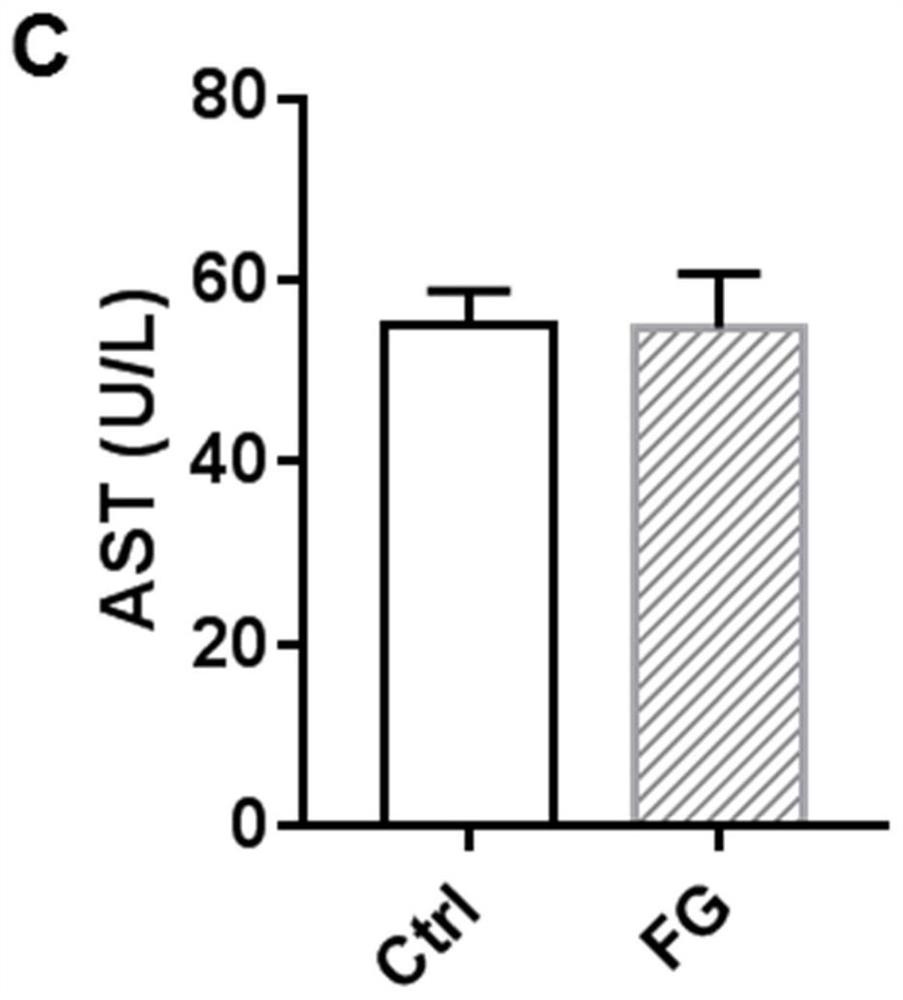
[0038] 10mg / kg therapeutic dose of FG4592 treated for 18 days had no toxic side effects on mice.
[0039] 1) Experimental materials
[0040] The C57BL / 6 mice used in the present invention were purchased from the Institute of Model Animals of Nanjing University; FG4592 was purchased from Selleck Company, and the 50 mg / mL mother liquid was prepared in DMSO and stored in a refrigerator at -80 degrees.
[0041] 2) Experimental method
[0042]Twelve male C57BL / 6 mice (7 weeks old, weighing 20-24g) were raised in an SPF-grade barrier environment in the Experimental Animal Center of Nanjing Medical University. The animals ate freely and maintained a circadian rhythm of 12 hours of light and 12 hours of darkness. After one week of adaptive feeding, they were randomly divided into control group (n=6) and FG4592 (FG) group (n=6). The mice in the FG group were treated with FG4592 (10 mg / kg) by intraperitoneal injection for 18 consecutive days; the mice in the control group were given t...
Embodiment 2
[0046] FG4592 promotes angiogenesis in renal tissue of ischemia-reperfusion mice.
[0047] 1) Experimental materials
[0048] The HIF1α antibody used in the present invention was purchased from Abcam; the VEGFA, VEGFR1, and GAPDH antibodies were purchased from ProteinTech; the CD31 antibody was purchased from CST; the HRP-labeled GoatAnti-Rabbit secondary antibody was purchased from Beyond Biotechnology Co., Ltd.; immunohistochemical reagents The box was purchased from Beijing Zhongshan Jinqiao Company; other biological materials and chemical materials were the same as in Example 1.
[0049] 2) Experimental method
[0050] Establishment of animal model and administration method
[0051] Thirty-six male C57BL / 6 mice (7 weeks old, weighing 20-24g) were raised in an SPF-grade barrier environment in the Experimental Animal Center of Nanjing Medical University. The animals ate freely and maintained a circadian rhythm of 12 hours of light and 12 hours of darkness. After one week ...
Embodiment 3
[0061] FG4592 promotes MAEC angiogenesis in mouse vascular endothelial cells.
[0062] 1) Experimental materials
[0063] Mouse vascular endothelial cells MAEC were purchased from ATCC; β-Actin antibody was purchased from CST; dual luciferase reporter gene detection kit was purchased from Promega; Lipofectamine 2000 was purchased from Invitrogen; Matrigel was purchased from Corning.
[0064] 2) Experimental method
[0065] Cell culture and drug treatment
[0066] Mouse vascular endothelial cells MAEC were cultured in DMEM medium containing 10% fetal bovine serum, containing 100 U / mL penicillin and 0.1 mg / mL streptomycin, and the culture conditions were 37°C, 5% carbon dioxide and 95% air. When the cell density was 90%, trypsinized and subcultured, and seeded in 6-well plates. On the next day, the FG4592 stock solution was diluted into serum-free medium to the final concentration (5 and 10 μg / mL, 0.1% DMSO), and the control cells were treated with serum-free medium containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com