Primer-probe, kit and rapid detection method for detecting novel coronavirus
A coronavirus, primer probe technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of nucleic acid instability, no primer-probe screening rules, and mismatching detection systems of extraction reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The present invention provides a primer and a probe for detecting the S gene of the novel coronavirus based on recombinase polymerase amplification technology, and after a large number of experimental verifications, it is finally determined that the nucleotide sequences shown in SEQ ID NO.1 and SEQ ID NO.2 are selected The upstream primer and downstream primer, and the probe containing the nucleotide sequence shown in SEQ ID NO.3. Among them, in the nucleotide sequence shown in SEQ ID NO: 3, the 32nd base from the 5' end is labeled with a FAM luminescent group, the 33rd base is connected with an abasic site, and the 35th base is Mark the quenching group BHQ1, the sequence is shown in Table 1. Sangon Bioengineering (Shanghai) Co., Ltd. was entrusted to synthesize the primer probes as shown in Table 1.
[0035] Table 1 Summary of primers and probes for detection of novel coronavirus E gene RPA
[0036]
[0037] (2) Specificity verification experiment
[0038] Select...
Embodiment 2
[0049] Establish the RT-RPA detection method that is used for novel coronavirus, it comprises the steps:
[0050] The sample to be tested can be serum, whole blood, plasma, throat swab, feces as a 10% liquid solution, and a small amount of nucleic acid release agent is mixed with the sample to be tested at 1:1 to extract the nucleic acid in the sample to be tested.
[0051] The RPA primer probe concentration and reaction system are as follows: Among them, the RT-RPA kit used is a fluorescent RNA constant temperature rapid amplification kit.
[0052] 1) Add 29 μL A buffer to each dry powder tube by the pipetting mechanism;
[0053] 2) Add 5 μL of primer-probe mixture (2 μL of the upstream primer shown in Example 1, 2 μL of the downstream primer shown in Example 1, and 1 μL of the probes at a concentration of 10 μM).
[0054] 3) Add 13.5 μL of the micro-nucleic acid releasing agent and the sample into the reaction tube by the pipetting mechanism.
[0055] 4) Add 2.5 μL of B buf...
Embodiment 3
[0060] Embodiment 3 repeatability verification experiment
[0061] According to the method described in Example 2, RT-RPA amplification was performed on the new coronavirus indoor quality control products S2 (median value) and S1 (low value) (diluted with PBS), and each sample was repeated three times for verification. The repeatability of this method.
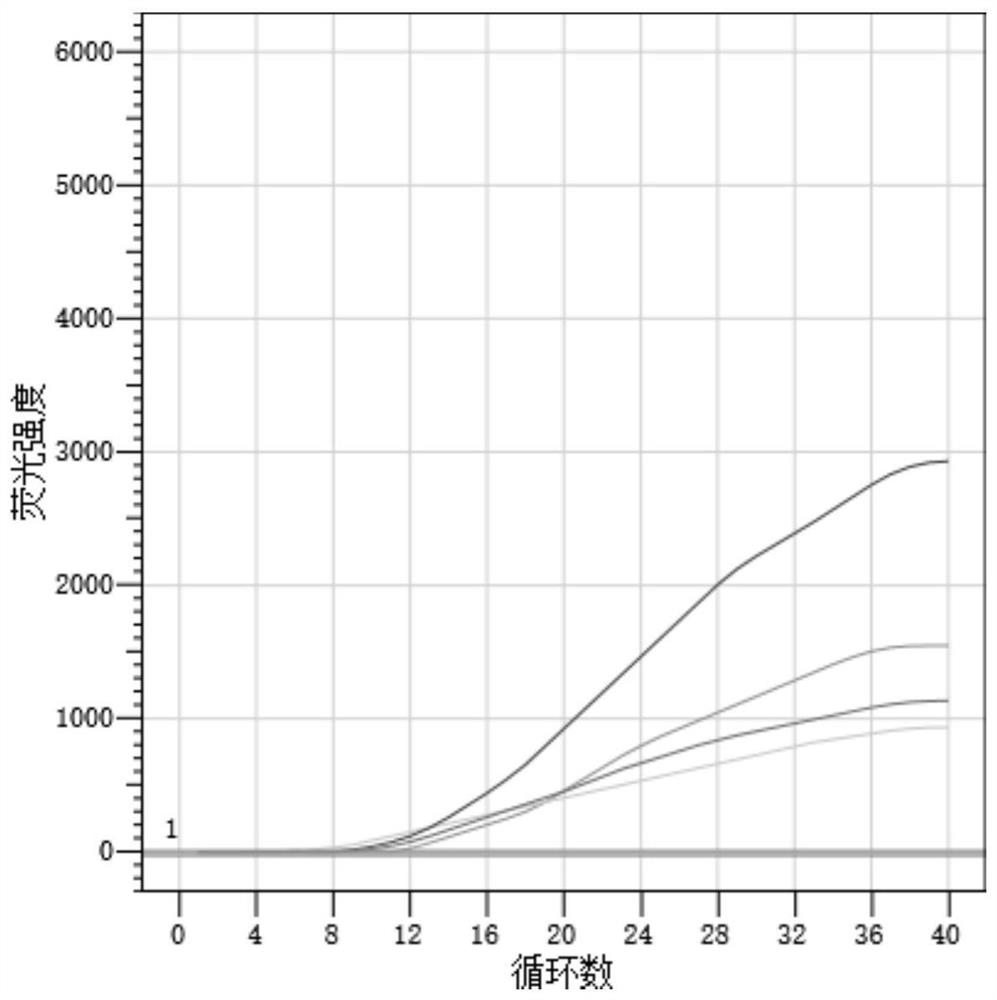
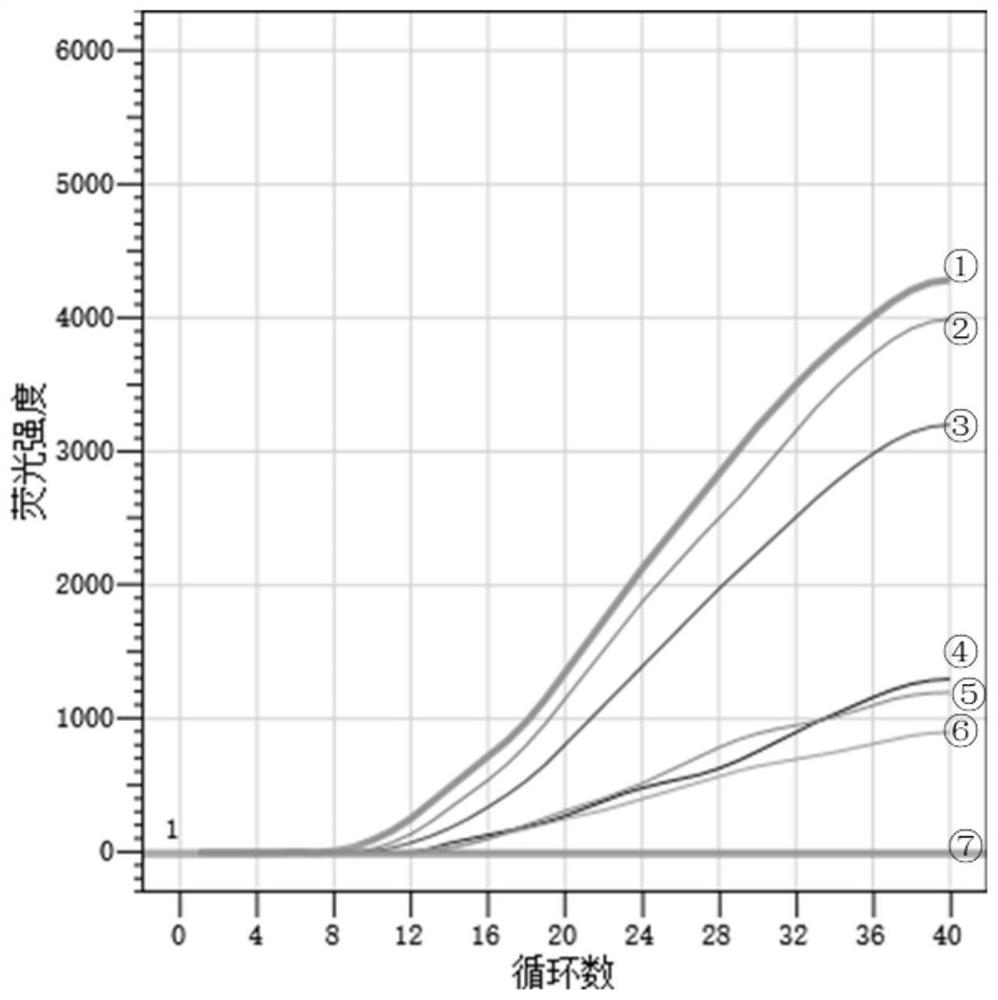
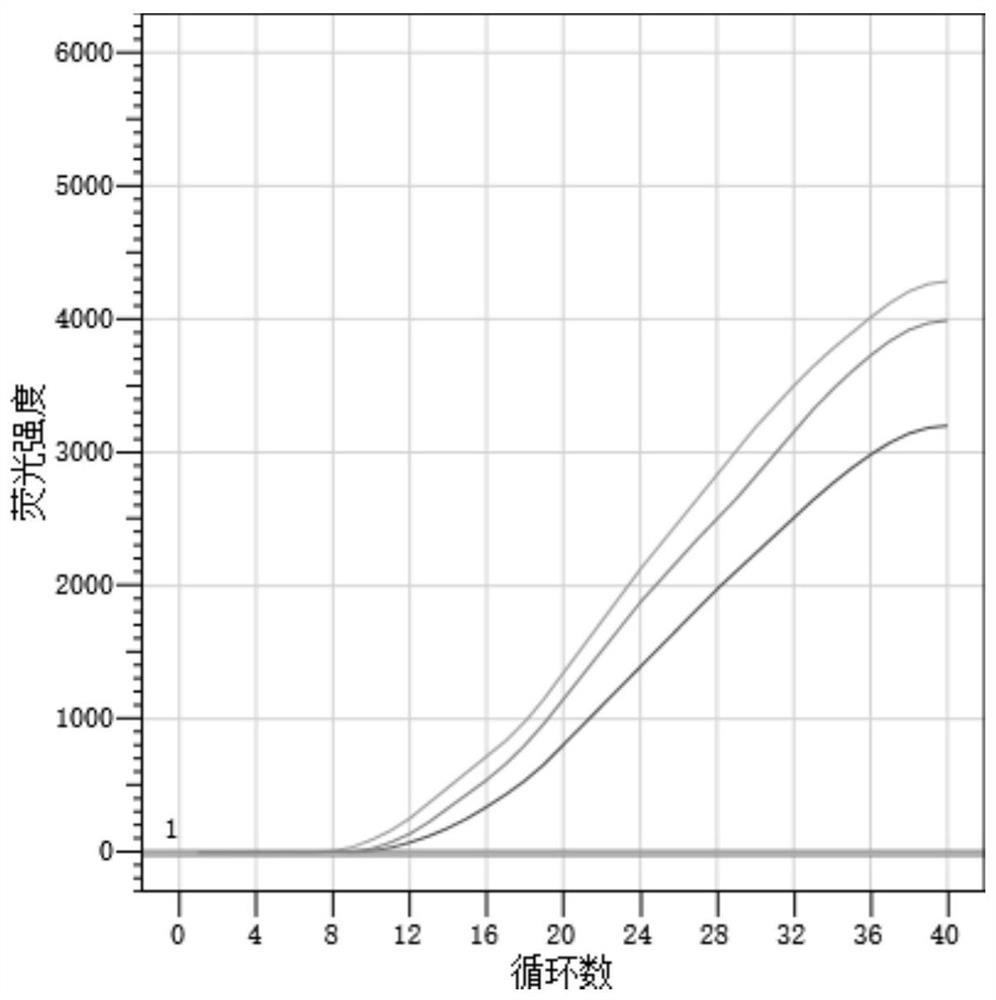
[0062] Result table 2, the amplification figure is shown in figure 2 , the results show that the new coronavirus indoor quality control products S2 (median value) and S1 (low value) have good repeatability in three experiments.
[0063] Table 2 Repeatability verification experiment of RPA detection of novel coronavirus E gene
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com