Synthetic method of loxoprofen sodium process impurity
A technology for loxoprofen sodium and process impurities, which is applied in the field of synthesis of loxoprofen sodium process impurities, and can solve the problems of loxoprofen sodium without open literature research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of step 1. formula III compound:
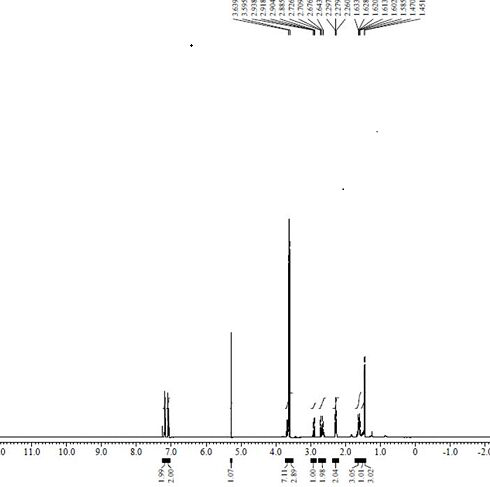
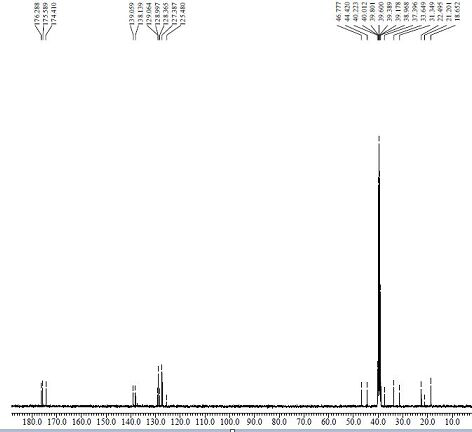
[0033] Put 275ml of anhydrous methanol into a 1000ml three-necked flask, add the compound of formula II (2-methoxycarbonylcyclopentanone) (31.93g, 224.6mmol), add sodium methoxide (34.67g, 641.71mmol) at 20~30℃, and stir After 10 minutes, cool down to 0~5°C, add dropwise the mixture of the compound of formula I (methyl p-bromomethylisophenylpropionate) (55.00g, 213.9 mmol) and 100ml of methanol, and keep the temperature at 0~5°C for reaction 5h, TLC spot plate central control (developing solvent: dichloromethane: methanol = 10:1), showed that there is no remaining raw material point, concentrated under reduced pressure and evaporated to dryness at 45°C, added 100ml of dichloromethane to dissolve, and washed with 50ml of water. The dichloromethane layer was obtained, which was concentrated and evaporated to dryness to obtain 66.43 g of a compound of formula III as a yellow oil, with a yield of 88.64% and an HPLC purity o...
Embodiment 2
[0037] The preparation of step 1. formula III compound:
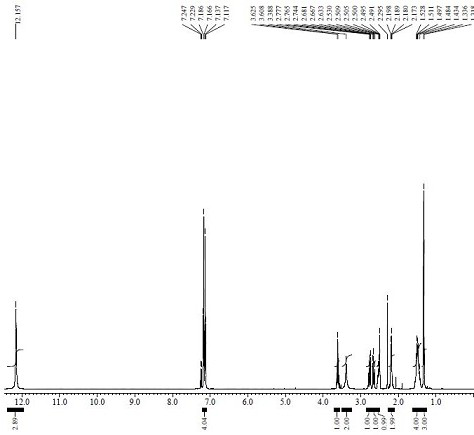
[0038] Put 275ml of anhydrous methanol into a 1000ml three-necked flask, add the compound of formula II (2-methoxycarbonylcyclopentanone) (31.93g, 224.6mmol), add sodium methoxide (23.11g, 427.80mmol) at 20~30℃, and stir 10min, cool down to 0~5°C, add dropwise the mixture of compound of formula I (methyl p-bromomethylisophenylpropionate) (55.00g, 213.9mmol) and 100ml methanol, after dropping, raise the temperature to 45~50°C and keep it warm Reaction for 3 hours, TLC spot plate central control (developing agent: dichloromethane: methanol = 10:1), showing that there is no remaining raw material point, concentrated under reduced pressure and evaporated to dryness at 45°C, added 100ml of dichloromethane to dissolve, washed with 50ml of water Separated to obtain a dichloromethane layer, concentrated and evaporated to dryness to obtain 62.62 g of a compound of formula III as a yellow oil, with a yield of 83.55% and an HPLC p...
Embodiment 3
[0042] The preparation of step 1. formula III compound:
[0043] Put 275ml of anhydrous methanol into a 1000ml three-necked flask, add the compound of formula II (2-methoxycarbonylcyclopentanone) (31.93g, 224.6mmol), add sodium methoxide (40.45g, 748.80mmol) at 20~30℃, and stir 10min, cool down to 0~5°C, add dropwise the mixture of compound of formula I (methyl p-bromomethylisophenylpropionate) (55.00g, 213.9mmol) and 100ml methanol, after dropping, raise the temperature to 5~10°C and keep it warm Reaction for 4 hours, TLC spot plate central control (developing solvent: dichloromethane: methanol = 10:1), showing that there is no remaining raw material point, concentrated under reduced pressure and evaporated to dryness at 45°C, added 100ml of dichloromethane to dissolve, washed with 50ml of water The dichloromethane layer was obtained by separation, and concentrated and evaporated to dryness to obtain 66.30 g of the compound of formula III as a yellow oil, with a yield of 88.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com