Berberine hydrochloride dimer as well as preparation method and application thereof
A technology of berberine hydrochloride and dimer, applied in the field of medicine, can solve the problems of less dimer, difficult synthesis of dimer, restricted compound research, etc., and achieves strong druggability, good antitumor activity, and great development value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
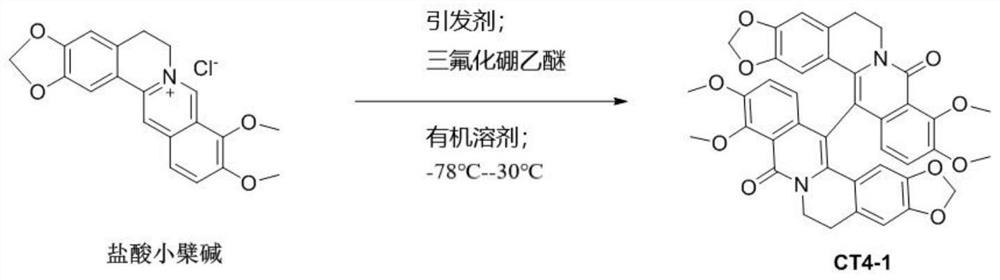
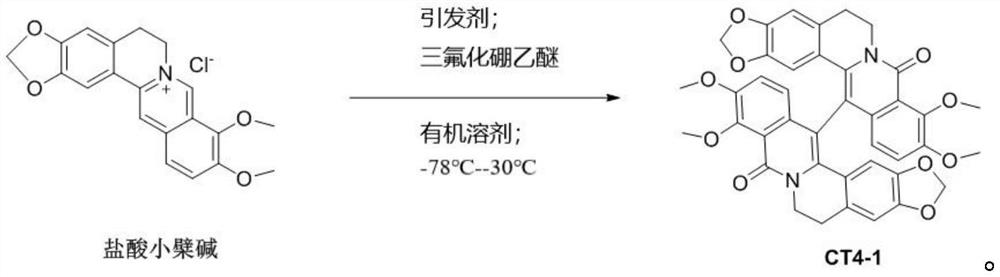
[0036]
[0037] Take 371mg, 1mmol of berberine hydrochloride, dissolve it in 10mL of dichloromethane, add 430mg, 1mmol of [bis(trifluoroacetoxy) iodine]benzene, 282mg, 2mmol of boron trifluoride ether at -78°C, and react 4 hours, obtain reaction solution;
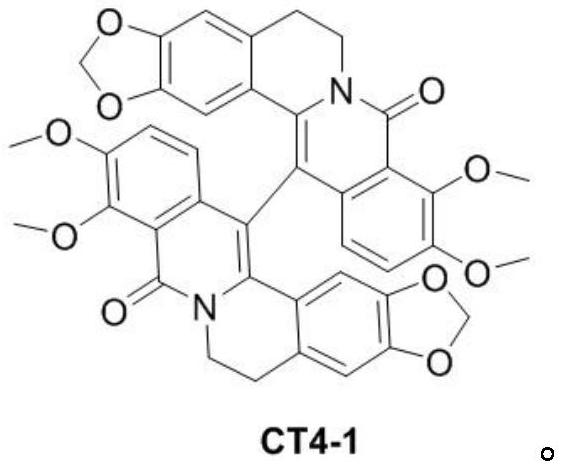
[0038] Use 60mL of ethyl acetate to extract the reaction solution, collect the organic phase, then dry the organic phase with sodium sulfate, filter and concentrate the organic phase, and then separate it through a silica gel column. The eluent is petroleum ether with a volume ratio of 3:1 to 1:1 Ethyl acetate mixture was separated to obtain 420 mg of the compound as a light yellow solid with a yield of 80 wt%.
Embodiment 2
[0040] The difference from Example 1 is that the reaction parameters are different.
[0041] Take 371mg, 1mmol of berberine hydrochloride, dissolve it in 10mL of tetrahydrofuran, and add 430mg, 1mmol of [bis(trifluoroacetoxy)iodo]benzene, 282mg, 2mmol of boron trifluoride ether at -78°C, and react for 4 hours , to obtain the reaction solution;
[0042] Use 60mL of ethyl acetate to extract the reaction solution, collect the organic phase, then dry the organic phase with sodium sulfate, filter and concentrate the organic phase, and then separate it through a silica gel column. The eluent is petroleum ether with a volume ratio of 3:1 to 1:1 Ethyl acetate mixture was separated to obtain 420 mg of the compound as a light yellow solid with a yield of 60 wt%.
Embodiment 3
[0044] The difference from Example 1 is that the reaction parameters are different.
[0045] Take 371mg, 1mmol of berberine hydrochloride, dissolve it in 10mL of dichloromethane, add 430mg, 1mmol of [bis(trifluoroacetoxy) iodine]benzene, 282mg, 2mmol of boron trifluoride ether at -30°C, and react 4 hours, obtain reaction solution;
[0046] Use 60mL of ethyl acetate to extract the reaction solution, collect the organic phase, then dry the organic phase with sodium sulfate, filter and concentrate the organic phase, and then separate it through a silica gel column. The eluent is petroleum ether with a volume ratio of 3:1 to 1:1. Ethyl acetate mixture was isolated to obtain 420 mg of the compound as a pale yellow solid, with a yield of 50 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com