Preparation and application of rare earth fluorescent material for detecting heavy metal Cu<2+>
A rare earth fluorescent material and heavy metal technology, applied in the field of preparation of rare earth fluorescent materials, can solve the problems of inaccurate detection results, low sensitivity, slow response time, etc., and achieve the effect of accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
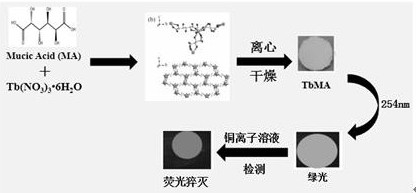
[0027] refer to figure 1 , a heavy metal Cu 2+ Preparation and application of rare earth fluorescent materials for ion detection, the steps are as follows:
[0028] S1, select raw materials, terbium nitrate hexahydrate and mucus acid;
[0029] S2, mucus acid is dissolved in distilled water, stirring;
[0030] S3, adding potassium hydroxide solution to the solution obtained in S2, stirring;
[0031] S4, adding terbium nitrate hexahydrate solution to the solution obtained in S3, stirring;
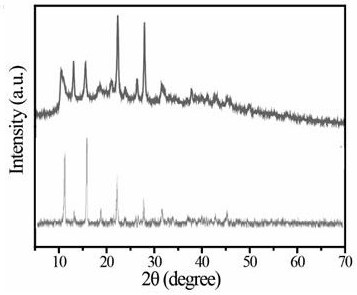
[0032] S5, stop stirring, let it stand for aging, centrifuge to collect the white precipitate, dry after washing to obtain a white sample of the rare earth fluorescent material;
[0033] S6, for the obtained rare earth fluorescent material for heavy metal Cu 2+ detection.
[0034] Further, the step S2 is stirring at room temperature for 10 minutes, and the steps S3 and S4 are both stirring at room temperature for 30 minutes;
[0035] Further, the standing time in step S5 is 24 hours, t...
Embodiment 2
[0046] The rare earth fluorescent material of the present invention is used as a fluorescent probe to detect heavy metal Cu 2+ ion.
[0047] Optional:
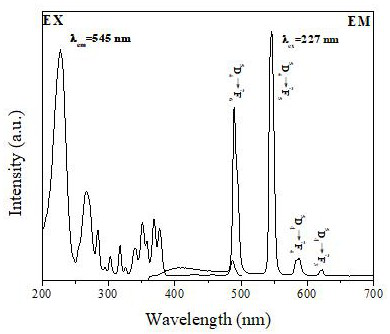
[0048] Figure 4 It shows that the rare earth fluorescent material is immersed in 1×10 at 298 K -2 The emission spectra of M different metal ions, the inset is a histogram of the emission intensity of the samples monitored at 545 nm. The excellent luminescent properties of rare-earth fluorescent materials make it suitable for common metal ions Sr 2+ 、Ba 2+ , Mg 2+ , Mn 2+ 、Na + , Ca 2+ 、K + , Zn 2+ 、Cr 3+ 、Ni 2+ 、Co 2+ 、Cu 2+ It has potential application value in detection. Figure 4 For rare earth fluorescent materials at 298 K, 1×10 -2 Emission spectra of M after immersion in various metal ions. The results showed that Sr 2+ 、Ba 2+ , Mg 2+ , Mn 2+ 、Na + , Ca 2+ 、K + , Zn 2+ 、Cr 3+ 、Ni 2+ 、Co 2+ The effect of metal ions on the photoluminescence intensity of rare earth fluorescent materials is not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com