Low-melting-point anti-dripping nylon 6 resin and preparation method thereof
An anti-droplet, low-melting-point technology, applied in the field of copolymerized low-melting-point nylon 6 resin, can solve problems such as scarcity of research work, uneven dispersion, migration of functional components, etc., and achieve expanded application range, fast burning speed, and heat release Falling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (a) Preparation of functional segments containing unsaturated double bonds
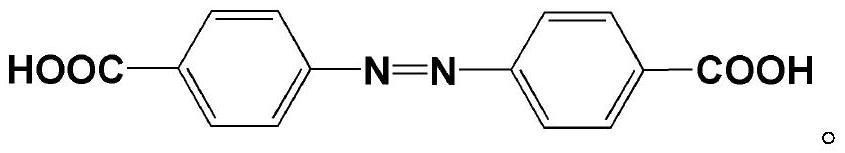
[0029] In parts by weight, take respectively 20g of 4-nitrobenzoic acid (4-NA) and 50g of sodium hydroxide (NaOH), which are dropped into 250mL of deionized water and stirred evenly, then heated in a water bath until fully dissolved; Subsequently, a certain amount of D-(+) glucose aqueous solution (100g / 150mL) was slowly added dropwise to obtain a brownish-black solution, and after 3 hours, it was filtered to obtain a bright brown precipitate; finally, the obtained precipitate was redissolved in deionized Aqueous solution, and add 25g of acetic acid for acidification, after the reaction is complete, after filtration, washing, precipitation and drying, a functional chain segment containing unsaturated double bonds is obtained, and its structural formula is:
[0030]
[0031] (b) Preparation of nylon 6 segments containing dicarboxylic groups
[0032] In parts by weight, the caprolactam of 200...
Embodiment 2
[0037] (a) Preparation of functional segments containing unsaturated double bonds
[0038] In parts by weight, take respectively 15g of 4-nitrobenzoic acid (4-NA) and 50g of sodium hydroxide (NaOH), which are dropped into 225mL of deionized water and stirred evenly, then heated in a water bath until fully dissolved; Subsequently, a certain amount of D-(+) glucose aqueous solution (100g / 150mL) was slowly added dropwise to obtain a brownish-black solution, and after 3 hours, it was filtered to obtain a bright brown precipitate; finally, the obtained precipitate was redissolved in deionized Aqueous solution, and add 25g of acetic acid for acidification, after the reaction is complete, obtain a functional segment containing unsaturated double bonds after filtration, washing, precipitation, and drying, and its structural formula is the same as that of Example 1.
[0039] (b) Preparation of nylon 6 segments containing dicarboxylic groups
[0040]In parts by weight, 200g of caprolac...
Embodiment 3
[0045] (a) Preparation of functional segments containing unsaturated double bonds
[0046] In parts by weight, take respectively 20g of 4-nitrobenzoic acid (4-NA) and 70g of sodium hydroxide (NaOH), which are dropped into 225mL of deionized water and stirred evenly, and heated in a water bath until fully dissolved; Subsequently, a certain amount of D-(+) glucose aqueous solution (100g / 150mL) was slowly added dropwise to obtain a brownish-black solution, and after 5 hours, it was filtered to obtain a bright brown precipitate; finally, the obtained precipitate was redissolved in deionized aqueous solution, and 35g of acetic acid was added for acidification, and after the reaction was complete, a functional segment containing an unsaturated double bond was obtained after filtration, washing, precipitation, and drying, and its structural formula was the same as in Example 1.
[0047] (b) Preparation of nylon 6 segments containing dicarboxylic groups
[0048] In parts by weight, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com