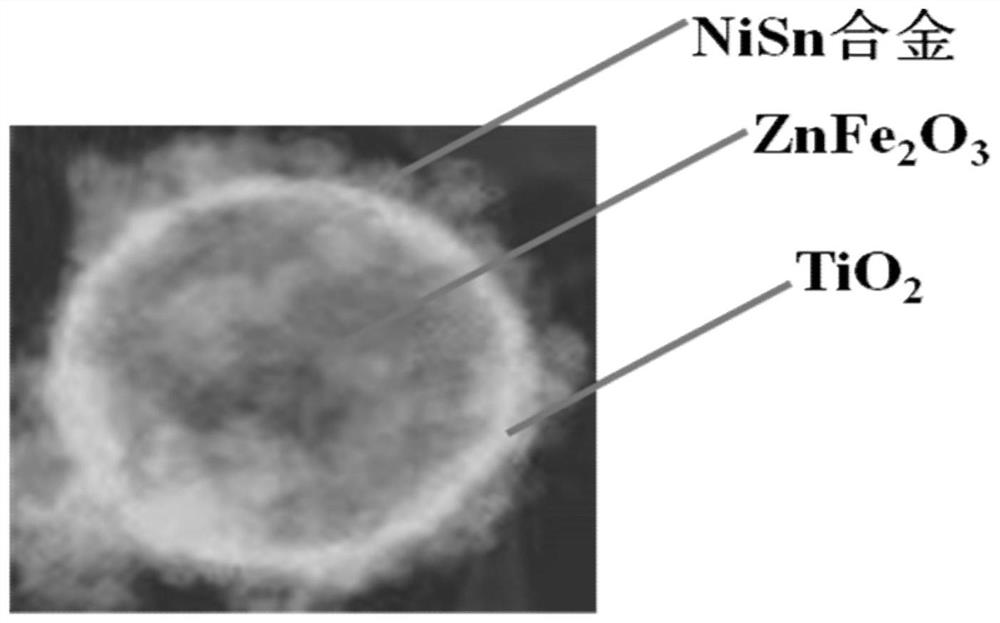
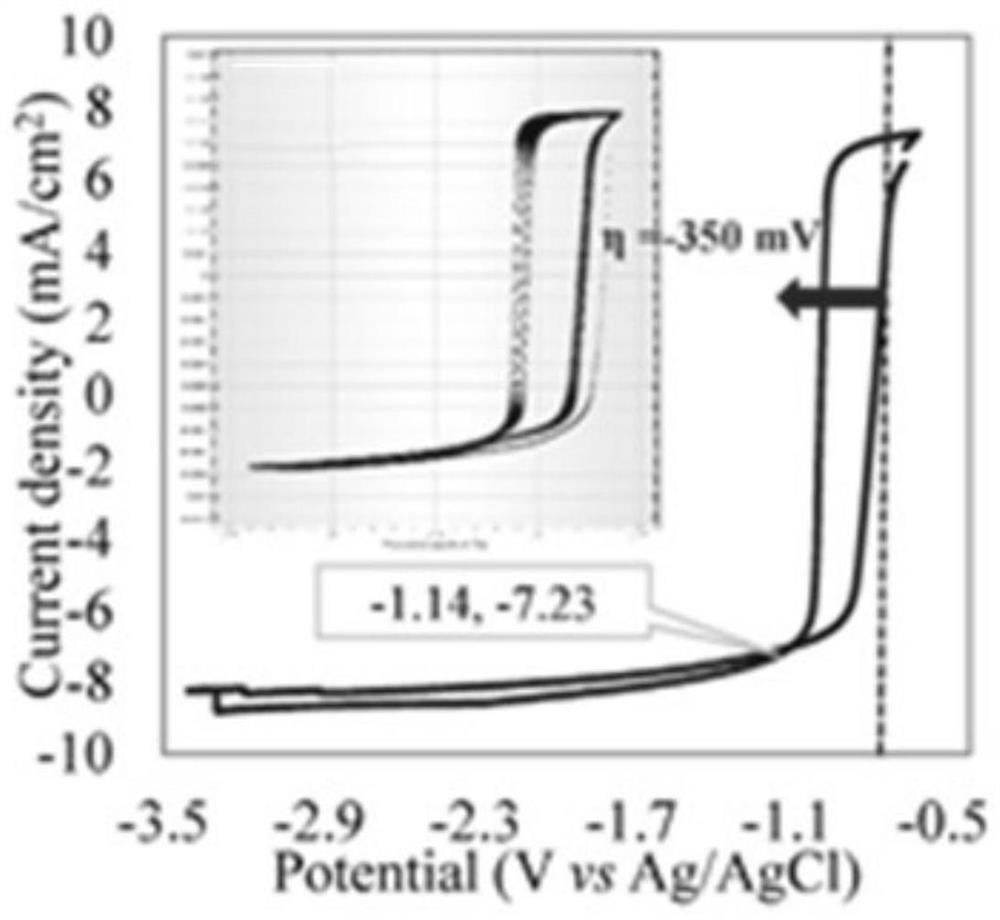
A kind of nisn/tio 2 @znfe 2 o 4 Electrocatalyst and its preparation method
An electrocatalyst, znfe2o4 technology, applied in the direction of electrodes, electrolysis components, electrolysis process, etc., to achieve the effect of promoting uniform dispersion, reducing the probability, and excellent conductive electronic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Porous spherical nano-ZnFe 2 o 4 Synthesis
[0029] 2.975g (0.01mol) of Zn(NO 3 ) 2 ·6H 2 O, 8.081g (0.02mol) of Fe(NO 3 ) 3 9H 2 O and 12.608g (0.06mol) citric acid were dissolved in 60mL of deionized water, 0.8mL of PEG 400 was added, stirred for 30 minutes to dissolve, the mixture was transferred to a 100mL stainless steel autoclave, and reacted at 180°C for 12h. The reaction kettle was naturally cooled to room temperature, and the obtained product was washed three times with distilled water and absolute ethanol, dried in a vacuum oven at 80°C, and roasted in a muffle furnace at 500°C for 3h with porous spherical nano-ZnFe 2 o 4 .
[0030] (2) Porous TiO 2 @ZnFe 2 o 4 Synthesis
[0031] Using the sol-gel method. Dissolve 17mL butyl titanate in 22mL absolute ethanol, add 0.68mL PEG-400 and 0.1667g porous nano ZnFe 2 o 4 , add dropwise a mixture of 22mL of absolute ethanol, 3.6mL of glacial acetic acid and 3.6mL of deionized water under stirring, hy...
Embodiment 2
[0037] (1) Porous spherical nano-ZnFe 2 o 4 Synthesis
[0038] 2.975g (0.01mol) of Zn(NO 3 ) 2 ·6H 2 O, 8.081g (0.02mol) of Fe(NO 3 ) 3 9H 2 O and 12.608g (0.06mol) citric acid were dissolved in 60mL of deionized water, 0.8mL of PEG 400 was added, stirred for 30 minutes to dissolve, the mixture was transferred to a 100mL stainless steel autoclave, and reacted at 180°C for 12h. The reaction kettle was naturally cooled to room temperature, and the obtained product was washed three times with distilled water and absolute ethanol, dried in a vacuum oven at 80°C, and roasted in a muffle furnace at 500°C for 3h with porous spherical nano-ZnFe 2 o 4 .
[0039] (2) Porous TiO 2 @ZnFe 2 o 4 Synthesis
[0040] Using the sol-gel method. Dissolve 17mL butyl titanate in 22mL absolute ethanol, add 0.68mL PEG-400 and 0.1237g porous nano ZnFe 2 o 4 , add dropwise a mixture of 22mL of absolute ethanol, 3.6mL of glacial acetic acid and 3.6mL of deionized water under stirring, hy...
Embodiment 3
[0046] (1) Porous spherical nano-ZnFe 2 o 4 Synthesis
[0047] 2.975g (0.01mol) of Zn(NO 3 ) 2 ·6H 2 O, 8.081g (0.02mol) of Fe(NO 3 ) 3 9H 2 O and 12.608g (0.06mol) citric acid were dissolved in 60mL of deionized water, 0.8mL of PEG 400 was added, stirred for 30 minutes to dissolve, the mixture was transferred to a 100mL stainless steel autoclave, and reacted at 180°C for 12h. The reaction kettle was naturally cooled to room temperature, and the obtained product was washed three times with distilled water and absolute ethanol, dried in a vacuum oven at 80°C, and roasted in a muffle furnace at 500°C for 3h with porous spherical nano-ZnFe 2 o 4 .
[0048] (2) Porous TiO 2 @ZnFe 2 o 4 Synthesis
[0049] Using the sol-gel method. Dissolve 17mL butyl titanate in 22mL absolute ethanol, add 0.68mL PEG-400 and 0.2105g porous nano ZnFe 2 o 4 , add dropwise a mixture of 22mL of absolute ethanol, 3.6mL of glacial acetic acid and 3.6mL of deionized water under stirring, hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com