Method for detecting residues 1, 2-dibromoethane and 1, 3-dibromopropane in homopiperazine
A detection method, the technology of dibromoethane, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve high accuracy, good detection effect, good precision and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Adopt gas chromatograph to measure according to gas chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0521), chromatographic condition is as follows:
[0202] Adopt capillary chromatographic column DB-624UI (30m×320μm×1.8μm); column temperature: start at 120°C, keep for 2min, raise the temperature to 180°C at a rate of 5°C / min, keep for 3min, then increase the temperature at 20°C / min Raise the temperature to 220°C and keep it for 0min;
[0203] The inlet temperature is 220°C, and the split ratio is 30:1;
[0204] The makeup gas flow rate is 30mL / min, and the detector (μECD) temperature is 260°C;
[0205] The carrier gas is nitrogen, and the flow rate of the carrier gas is 1mL / min;
[0206] The injection volume of the sample to be tested is 1μL, and the column flow rate is 1.5mL / min;
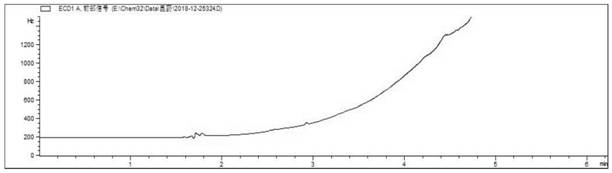
[0207] Obtain the chromatogram of 1,2-dibromoethane and 1,3-dibromopropane mixed standard control solution see Figure 15 shown.
[0208] Depend on Figure 15 It ...
Embodiment 2
[0210] Adopt gas chromatograph to measure according to gas chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0521), chromatographic condition is as follows:
[0211] Adopt capillary chromatographic column DB-624UI (30m×320μm×1.8μm); column temperature: initial temperature is 120°C, keep for 2min, raise the temperature to 130°C at a rate of 5°C / min, keep for 6min;
[0212] The inlet temperature is 220°C, and the split ratio is 30:1;
[0213] The makeup gas flow rate is 30mL / min, and the detector (μECD) temperature is 260°C;
[0214] The carrier gas is nitrogen, and the flow rate of the carrier gas is 1mL / min;
[0215] The injection volume of the sample to be tested is 1μL, and the column flow rate is 1.5mL / min;
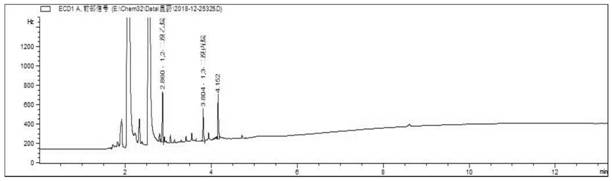
[0216] Obtain the chromatogram of 1,2-dibromoethane and 1,3-dibromopropane mixed standard control solution see Figure 16 shown.
[0217] Depend on Figure 16 It can be seen that under the chromatographic conditions of Example 2, the target pea...
Embodiment 3
[0219] Adopt gas chromatograph to measure according to gas chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0521), chromatographic condition is as follows:
[0220] Adopt capillary chromatographic column DB-624UI (30m×320μm×1.8μm); column temperature: the initial temperature is 120°C, keep it for 2min, raise the temperature to 130°C at the rate of 5°C / min, keep it for 6min, then increase the temperature at 20°C / min Raise the temperature to 220°C and keep it for 1min;
[0221] The inlet temperature is 220°C, and the split ratio is 30:1;
[0222] The makeup gas flow rate is 30mL / min, and the detector (μECD) temperature is 260°C;
[0223] The carrier gas is nitrogen, and the flow rate of the carrier gas is 1mL / min;
[0224] The injection volume of the sample to be tested is 1μL, and the column flow rate is 1.5mL / min;
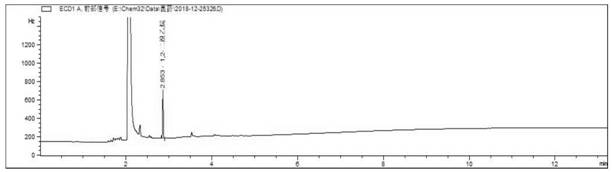
[0225] Obtain the chromatogram of 1,2-dibromoethane and 1,3-dibromopropane mixed standard control solution see Figure 17 shown.
[0226]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com