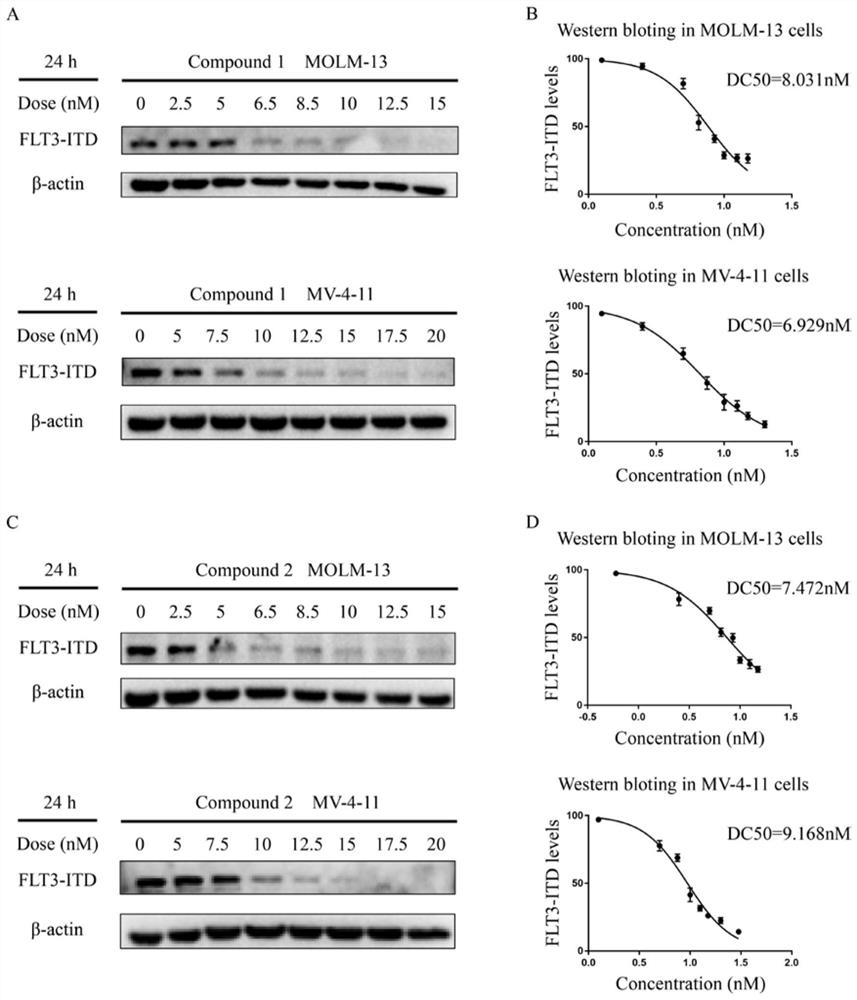
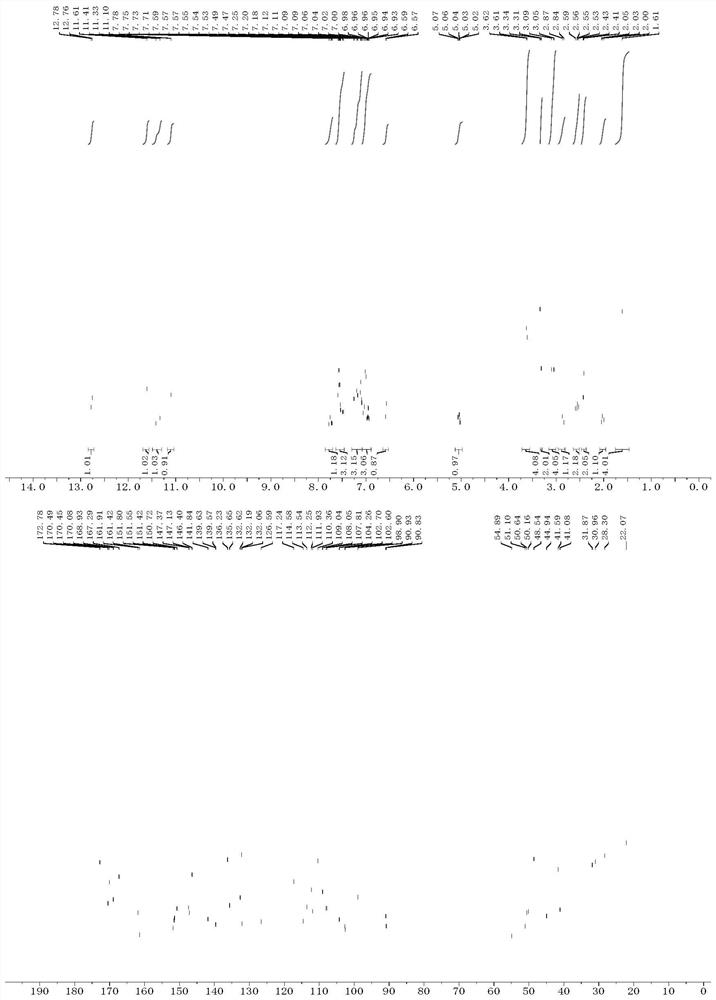
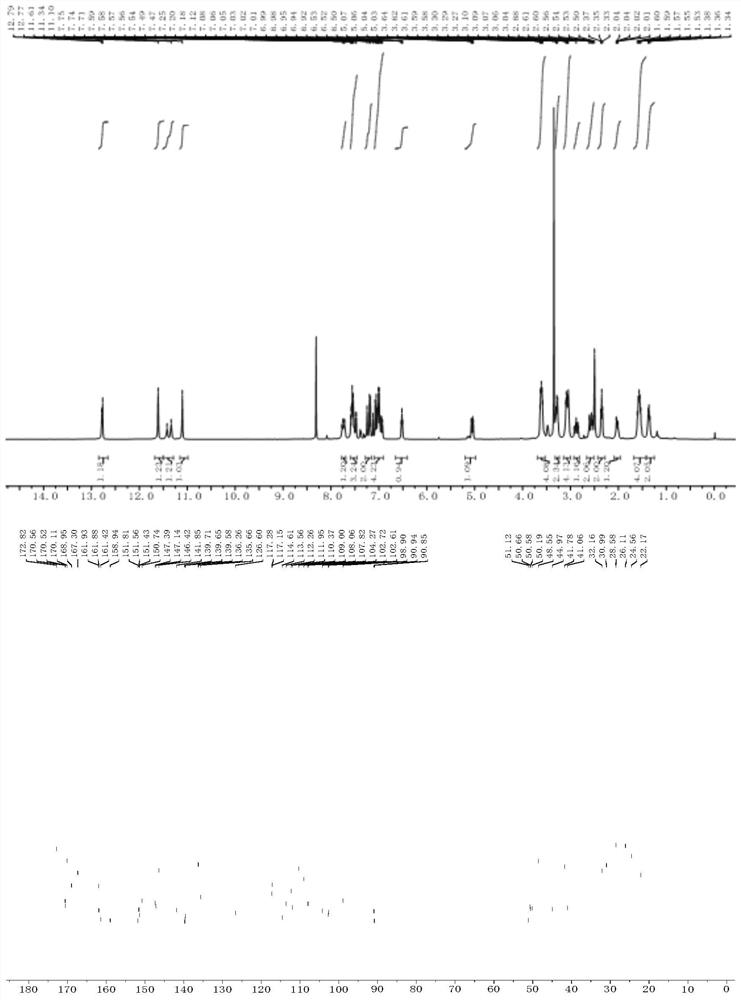
Proteolysis targeting chimera, and pharmaceutical composition and application thereof
A technology of proteolysis and proteolysis, which is applied in the field of proteolysis-targeted chimera and its pharmaceutical composition, can solve the problems of low treatment efficiency, limited reports, and large dosage, achieve good treatment effect and reduce the level of positive cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of compound 1 and 2
[0026] The synthetic route of compound 1 (n=4), 2 (n=5) and 14 (n=6) is as follows:
[0027]
[0028] The specific preparation steps of intermediate 4 in the technical route are: dissolving 5-fluoro-2-nitroaniline (4.68g, 30.0mmol) in dry 1-methyl-2-pyrrolidone (NMP, 150mL), and then Triethylamine (10.4 mL, 75.0 mmol) and 1-tert-butoxycarbonylpiperazine (6.15 g, 33.0 mmol) were added, and the mixture was heated to 110° C. for 15 h. The reaction solution was cooled to room temperature, poured into 150 mL of water, and a yellow precipitate appeared. Filter through a glass funnel under reduced pressure, and wash the filter cake twice with ether. The filter cake was collected and dried under vacuum to give compound 4 as a yellow solid (7.93 g, 82%).
[0029] Intermediate 4 was detected, and the detection data was: M.p.244.2-245.1℃; IR(KBr):3468,3335,2974,1676,1656,1618,1566,1474,1424,1370,1246,1222,1170,1120 ,1038cm ...
Embodiment 2
[0045] Example 2: Synthesis of Compound 19a-i
[0046] The synthetic route of compound 19a-i is as follows:
[0047]
[0048] The specific preparation steps of intermediate 18a in the technical route are: add intermediate 15 (273mg, 1.00mmol) to dry tetrahydrofuran (5mL), then add 3-bromopropionyl chloride A2a (514mg, 3.00mmol) to the above mixture in the liquid. The mixture was heated to reflux for 6h. After cooling to room temperature, the reaction solution was concentrated under reduced pressure. Then add saturated NaHCO 3 (50 mL), and extracted with dichloromethane (50 mL). The organic phase was collected and washed with Na 2 SO 4 dry. Filter and concentrate under reduced pressure to obtain the crude product, which is purified by silica gel column chromatography (CH 2 Cl 2 :CH 3 OH=60:1), finally obtained white solid compound 18a (290 mg, 71%).
[0049] Intermediate 15 was detected, and the detection data was: M.p.180.6-181.7℃; IR(KBr):3354,3208,3100,1767,169...
Embodiment 3
[0071] Example 3: Synthesis of Compounds 24a-c
[0072] The synthetic route of compound 24a-c is as follows:
[0073]
[0074] The preparation method of intermediate 21 in the technical route is the same as that of 11, and the obtained intermediate 21 is a purple-gray solid with a yield of 79%. Detect it, and its detection data is: M.p.>300℃; IR(KBr):3177,1725,1702,740cm -1 . 1 HNMR (400MHz, CDCl 3 )δ11.13(s, 1H), 8.27(d, J=7.9Hz, 1H), 7.92(d, J=7.3Hz, 1H), 7.58(t, J=7.6Hz, 1H), 5.16(dd, J=12.7,5.4Hz,1H),2.89(ddd,J=18.1,13.9,5.4Hz,1H),2.69–2.52(m,2H),2.18–1.90(m,1H). 13 C NMR (100MHz, CDCl 3 )δ172.7, 169.7, 166.0, 165.3, 145.4, 135.6, 133.2, 131.8, 123.2, 90.3, 49.1, 30.9, 21.8. HRMS (ESI) calculated for C 13 h 10 IN 2 o 4 + [M+H] + :384.9680, found 384.9682.
[0075] The specific preparation steps of intermediate 23a in the technical route are: adding CH to intermediate 8 (574mg, 1.20mmol) 2 Cl 2 (6mL), TFA (4mL), stirred at room temperature for 2h. The mixt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com