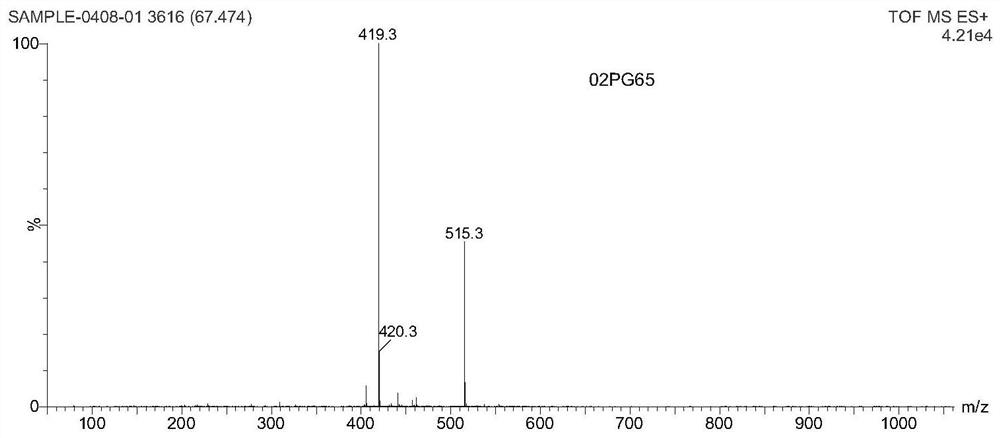
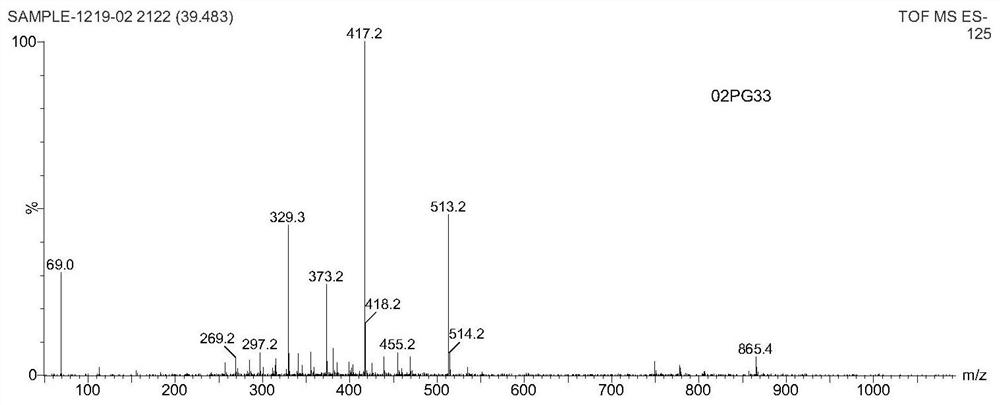
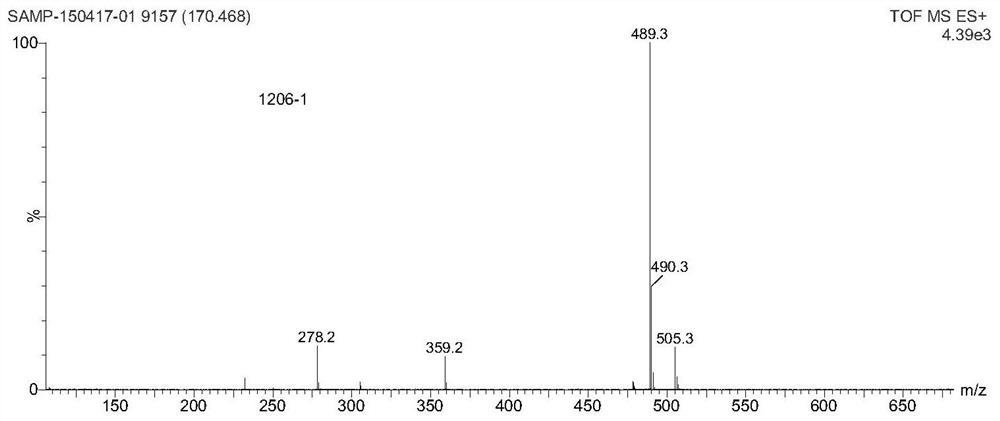
A kind of small molecule fluorescent probe and its preparation method and application
A fluorescent probe and small molecule technology, applied in the field of fluorescence imaging, can solve the problems of non-target specificity, high cost, and complicated operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of Intermediate 5
[0038] Intermediate 5-1 Synthesis: add 3.87g (2.0mmol) 4-(diethylamino)benzoic acid to a 100mL round-bottomed flask, add 60mL freshly distilled thionyl chloride and 0.5mL DMF, heat under reflux at 75°C, react for 4 hours, stop heating , recovering unreacted SOCl 2 , residual S°C l 2 It was evaporated under reduced pressure, and the crude product was recrystallized from ethyl acetate to obtain 0.38 g of intermediate 5-1 as white crystals with a yield of 90%.
[0039] Intermediate 5-2: Synthesis of : refer to the synthesis of intermediate 5-1, using 4-(dimethylamino)benzoic acid to synthesize intermediate 5-2 with a yield of 92%.
Embodiment 2
[0040] Example 2: Synthesis of Intermediate 2
[0041] Intermediate 2-1 Synthesis: 1.59 g (10.0 mmol) of 5-amino-1-naphthol and 2.4 g (11.0 mmol) of di-tert-butyl dicarbonate were placed in a 100 mL round-bottomed flask, 20 mL of tetrahydrofuran was added to dissolve, and then sodium bicarbonate was added. 1.25g (15.0mmol) and 10mL of water were reacted at room temperature, TLC detected the reaction progress (developing solvent: ethyl acetate: n-hexane=1:3, v:v), after the reaction was finished, the tetrahydrofuran solvent was evaporated under reduced pressure and added 100mL Distilled water, a large amount of black solid was precipitated, filtered, washed with a large amount of distilled water and n-hexane successively to obtain Intermediate 2-1 (black solid).
Embodiment 3
[0042] Example 3: Synthesis of Intermediate 3
[0043] Intermediate 3-1 Synthesis of: 25mL round-bottomed flask was fully dried before use, and purged with nitrogen, added magnetron, quickly added 0.12g (5mmol) of sodium hydride and 0.259g (1mmol) of intermediate 2-1, plugged with a rubber stopper, filled with Nitrogen protection, 0 ° C ice bath cooling, add 5 mL of dry DMF, stir for 30 minutes, slowly add 0.568 g (4 mmol) iodomethane dropwise at 0 ° C, react at 0 ° C to room temperature, TLC detects the reaction progress (developing solvent: ethyl acetate Ester: n-hexane=1:3, v:v), after the reaction, 20 mL of distilled water was added to quench the reaction, extracted with ethyl acetate (25 mL×3), the ethyl acetate layers were combined, washed with water (25 mL×3), saturated common salt Washed with water (10 mL×2), the organic layer was dried with anhydrous sodium sulfate, filtered, and the ethyl acetate was evaporated under reduced pressure to obtain the crude product, whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com