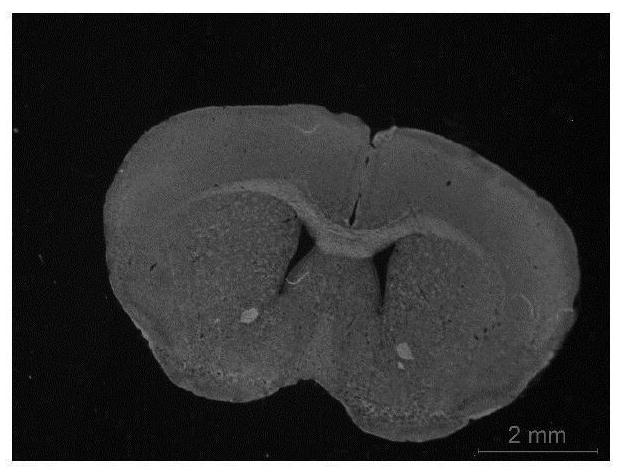
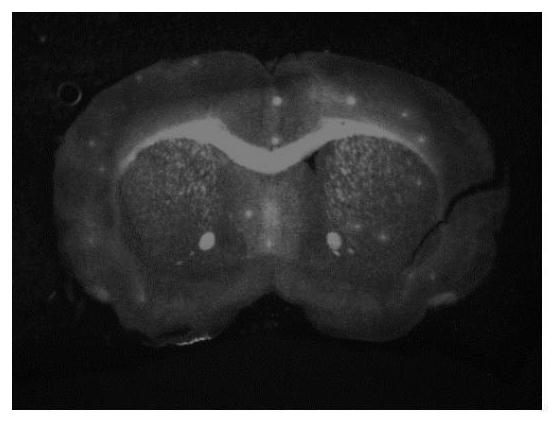
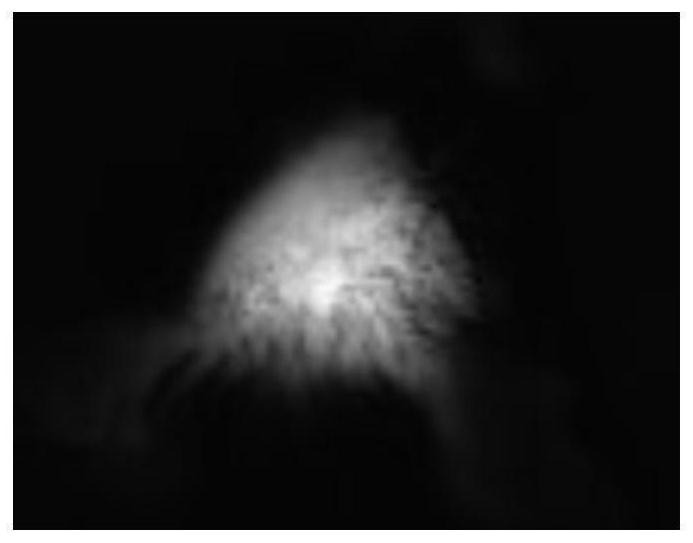
A near-infrared fluorescent small molecule probe and its preparation and application
A small-molecule probe and near-infrared technology, applied in the field of near-infrared imaging, can solve the problems of complex operation, lack of targeting specificity, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The method for preparing a near-infrared fluorescent small molecule probe is characterized in that the solvent described in the first step reaction is methanol, acetonitrile or tetrahydrofuran;
[0030] The method for preparing a near-infrared fluorescent small molecule probe is characterized in that the heating reaction temperature in the second step reaction is 70-120°C.
Embodiment 1
[0032] In the first step, 1.59 g (10 mmol) of 5-amino-1-naphthol (compound 1) was dissolved in 30 ml of methanol, 0.84 g of solid sodium bicarbonate was added, and 10 ml of methanol containing 1.42 g of methyl iodide was added dropwise with stirring The solution was first reacted at room temperature for half an hour, and then refluxed for 4 hours. After the reaction was completed, 5-methylamino-1-naphthol (Intermediate 2) was obtained after post-processing with a yield of 75%;
[0033] In the second step, 0.1 g of intermediate 2 was dissolved in 5 ml of DMF, and then 0.12 g of 5-diethylamino-2-nitrosophenol (compound 3) was added, and the reaction was heated at 70 ° C. After the reaction was completed, after-treatment The target product 4 was obtained with a yield of 55%; 1 H NMR (500MHz, Chloroform-d) δ 8.48 (d, J=9.3Hz, 1H), 8.20 (s, 1H), 7.67 (t, J=7.7Hz, 1H), 7.61 (d, J=9.1Hz) ,1H),7.55(d,J=7.6Hz,1H),6.67(dd,J=9.1,2.7Hz,1H),6.45(d,J=2.6Hz,1H),6.42(s,1H),3.49 (q,J=7.1Hz,4...
Embodiment 2
[0035] In the first step, 1.59 g (10 mmol) of 5-amino-1-naphthol (compound 1) was dissolved in 30 ml of acetonitrile, 0.84 g of solid sodium bicarbonate was added, and 10 ml of acetonitrile containing 1.42 g of methyl iodide was added dropwise with stirring The solution was first reacted at room temperature for half an hour, and then refluxed for 4 hours. After the reaction was completed, 5-methylamino-1-naphthol (intermediate 2) was obtained after post-processing with a yield of 83%;
[0036] In the second step, 0.1 g of intermediate 2 was dissolved in 5 ml of DMF, then 0.12 g of 5-diethylamino-2-nitrosophenol (compound 3) was added, and the reaction was heated at 100 ° C. After the reaction was completed, after-treatment The target product 4 was obtained with a yield of 66%; 1 H NMR (500MHz, Chloroform-d) δ 8.48 (d, J=9.3Hz, 1H), 8.20 (s, 1H), 7.67 (t, J=7.7Hz, 1H), 7.61 (d, J=9.1Hz) ,1H),7.55(d,J=7.6Hz,1H),6.67(dd,J=9.1,2.7Hz,1H),6.45(d,J=2.6Hz,1H),6.42(s,1H),3.49 (q, J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com