Method for synthesizing nitramine azide through phase-transfer catalysis
A technology of phase transfer catalyst and synthesis method, which is applied in the field of energetic material synthesis, can solve problems such as environmental pollution, restrictions on large-scale production and popularization of DIANP, difficulties in recycling and processing DMSO aqueous solution, etc., and achieve good large-scale production effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
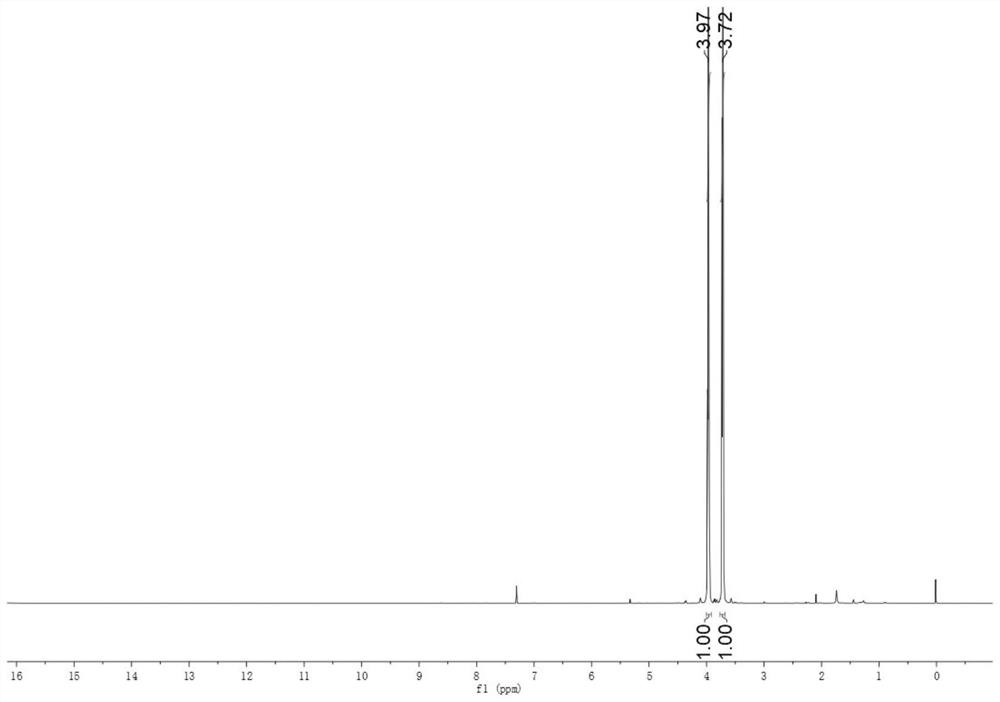
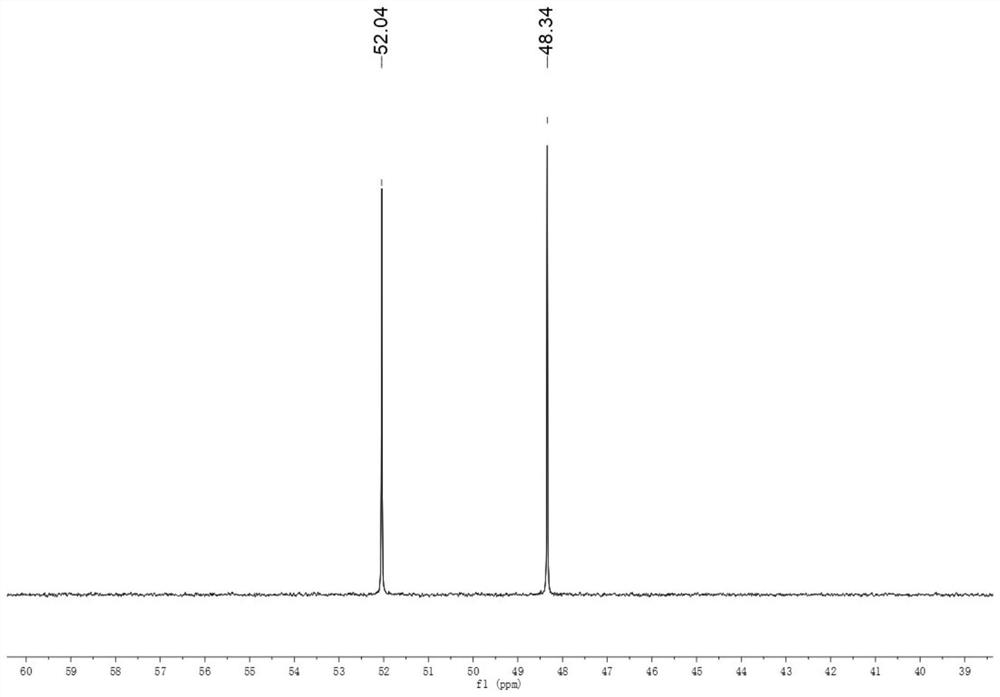
[0022] Add 2 g of Gena to a 250 mL flask, followed by 50 mL of CH 2 Cl 2 ; 1.19 g NaN 3 Add 50 mL of water and stir to dissolve, pour it into a flask together, and finally add 0.4 g of tetrabutylammonium bromide, and stir vigorously at room temperature for 6 h. After the reaction is finished, use a separatory funnel to separate the liquid. The lower liquid is the methylene chloride phase, the upper liquid is the water phase, and the tetrabutylammonium bromide in the water phase can be recycled. The lower layer liquid was dried with anhydrous sodium sulfate, rotary evaporated at 40 °C, and separated by column chromatography to obtain DIANP with a purity of 98.2% (HPLC, the same below).
Embodiment 2
[0024] Add 4 g of Gena to a 250 mL flask, followed by 50 mL of CH 2 Cl 2 ; 2.38 g NaN 3 Add 50 mL of water and stir to dissolve, pour it into a flask together, and finally add 0.35 g of tetraethylammonium bromide, pass it through a condensing reflux device at 40 °C, and stir vigorously for 10 h. After the reaction was completed, the liquid was separated with a separatory funnel, and the lower liquid was dried with anhydrous sodium sulfate, rotary evaporated at 40 °C, and separated by column chromatography to obtain DIANP with a purity of 98.7%.
Embodiment 3
[0026] Add 6 g of Gena to a 250 mL flask, followed by 50 mL of CHCl 3 ; 3.6 g NaN 3 Add 50 mL of water and stir to dissolve, pour them into the flask together, and finally add 0.57 g of benzyltriethylammonium bromide, at 60°C, pass through the condensing reflux device, and stir vigorously for 12 h. After the reaction, use a separatory funnel to separate the liquid, the lower layer is the chloroform phase, the upper layer is the water phase, and DIANP is dissolved in chloroform. The lower layer was dried with anhydrous sodium sulfate, concentrated by rotary evaporation at 40 °C, and separated by column chromatography to obtain DIANP with a purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com