A class of lipid droplet specific fluorescent probe and its synthesis method
A technology of fluorescent probe and synthesis method, which is applied in the field of lipid droplet-specific fluorescent probe and its synthesis, can solve the problems of narrow emission wavelength range and limited biological imaging application, and achieve broadened wavelength range, easy operation and wide application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-targeted fluorescent probe, the chemical reaction formula of which is as follows:
[0040]
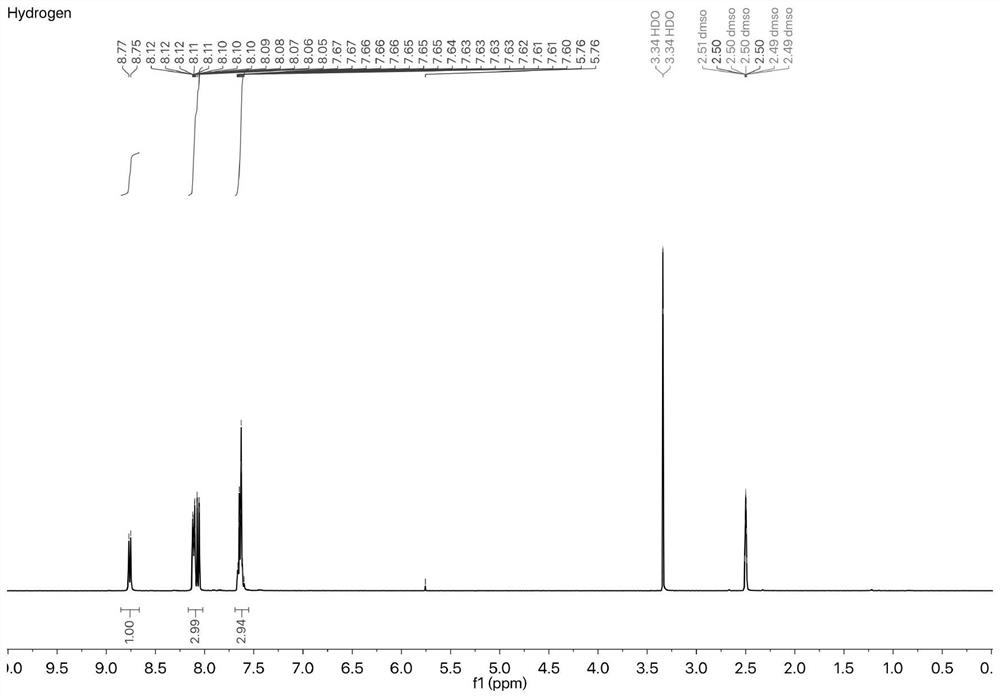
[0041]The specific steps are as follows: under the protection of argon, benzene borate (0.321g, 8.18mmol), 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (0.5g, 5mmol) and Bis(tri-tert-butylphosphine)palladium (0.0512 g, 1.95 mmol) was added to a 250 mL three-necked flask. Sonicated tetrahydrofuran (15 mL) and water (1.5 mL) were added, and the mixture was stirred at 75° C. for 24 hours under argon protection. Subsequently, it was concentrated, dissolved in dichloromethane and filtered. After further concentration, the product was separated by flash column chromatography to obtain 0.43 g of a reddish-brown product with a yield of 70.4%. figure 1 .
Embodiment 2
[0043] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-targeted fluorescent probe, the chemical reaction formula of which is as follows:
[0044]
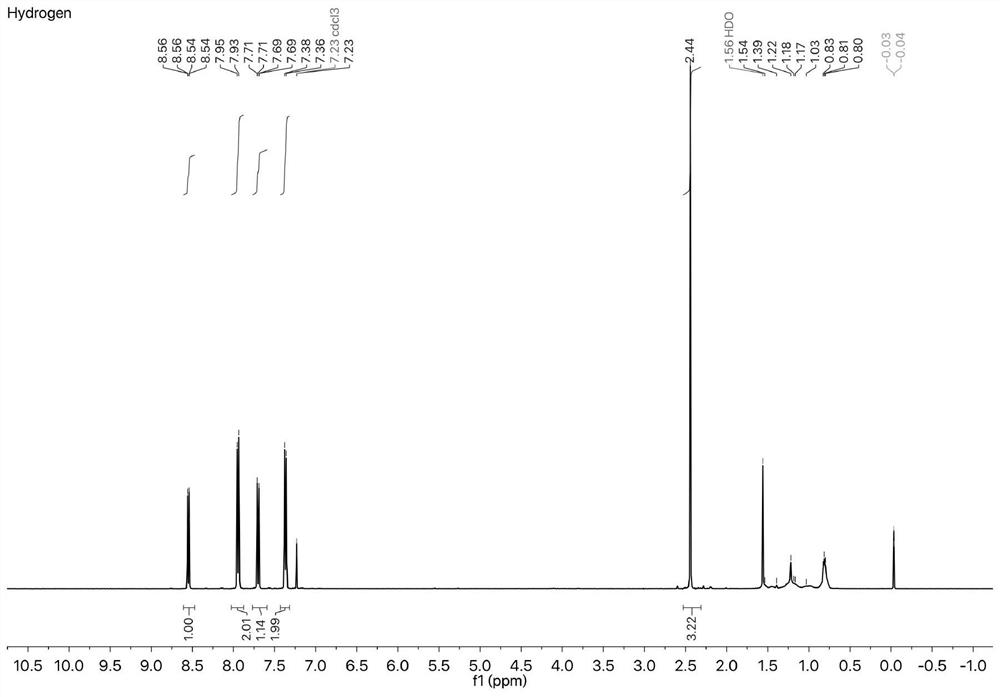
[0045] The specific steps are as follows: under the protection of argon, methylphenylboronic acid (0.352g, 7.45mmol), 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (0.5g, 5mmol) ), bis(tri-tert-butylphosphine)palladium (0.0512g, 1.95mmol) was added to a 250mL there-necked flask. Sonicated tetrahydrofuran (15 mL) and water (1.5 mL) were added, and the mixture was stirred at 75° C. for 24 hours under argon protection. After the reaction was completed, it was concentrated, dissolved in dichloromethane and filtered. After further concentration, the product was separated by flash column chromatography to obtain 0.49 g of a reddish-brown product with a yield of 75.3%. figure 2 .
Embodiment 3
[0047] A preferred embodiment of the present invention provides a method for synthesizing a lipid droplet-targeted fluorescent probe, the chemical reaction formula of which is as follows:
[0048]
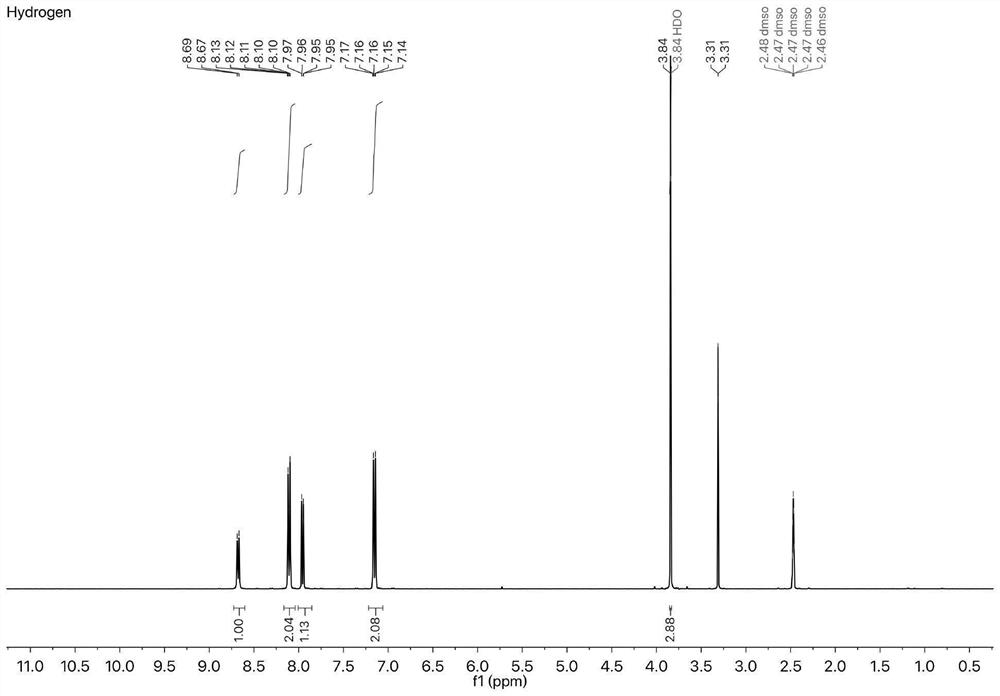
[0049] The specific steps are as follows: under the protection of argon, methoxybenzeneboronic acid (0.37g, 7.13mmol), 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (0.5g, 5mmol), bis(tri-tert-butylphosphine)palladium (0.0512g, 1.95mmol) was added to a 250mL there-necked flask. Sonicated tetrahydrofuran (15 mL) and water (1.5 mL) were added, and the mixture was stirred at 75° C. for 24 hours under argon protection. Subsequently, it was concentrated, dissolved in dichloromethane and filtered. After further concentration, the product was separated by flash column chromatography to obtain 0.46 g of a reddish-brown product with a yield of 67%. image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com