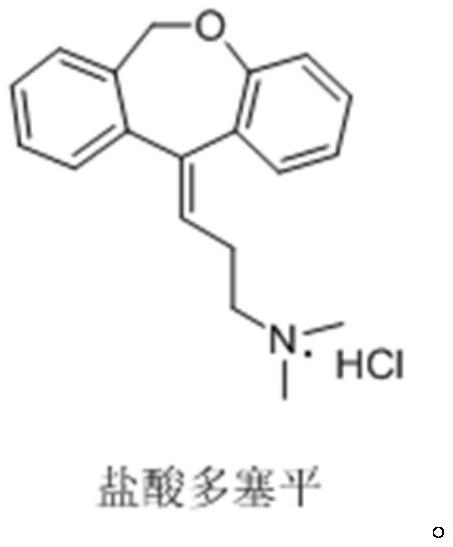
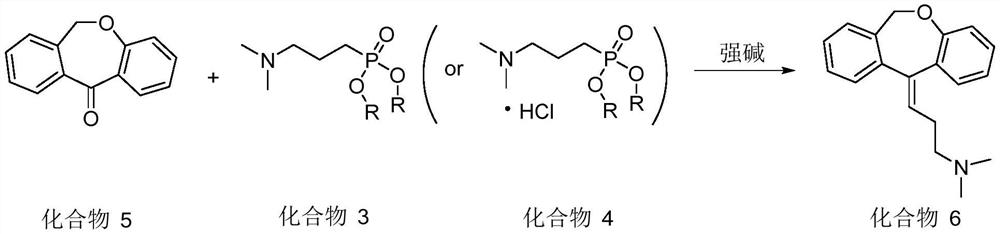
Synthesis method of doxepin hydrochloride
A technology of doxepin hydrochloride and a synthesis method, applied in the field of drug synthesis, can solve the problems of complex process, harsh synthesis conditions and the like, and achieve the effects of simple process, high repetition rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
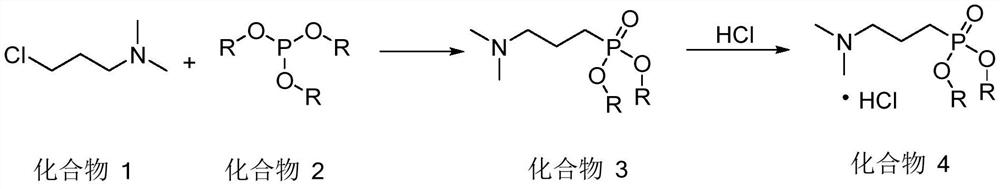
[0045] Slowly added triethyl phosphite (90.18g, 542.73mmol) into 3-chloro-1-(N,N-dimethyl)propylamine (60g, 493.38mmol), heated to 140°C, and stirred for 8h. After the reaction solution was cooled, it was quenched by adding water, extracted three times with ethyl acetate, and the organic phase was concentrated to dryness under reduced pressure. Distilled under reduced pressure to obtain 97.5 g of 3-(N,N-dimethyl)propyl diethyl phosphate (compound 3) with a yield of 88.5%.
[0046] 1 H NMR (400MHz,D 2 O)δ3.77–3.69(m,4H),3.01(q,J=7.0,6.2Hz,2H),2.60(s,6H),1.93(dq,J=12.0,6.4Hz,2H),1.66( dq,J=12.0,6.4Hz,2H),0.99(t,J=7.1Hz,6H). 13 CNMR (125MHz,D 2 O) δ61.30, 61.25, 57.74, 57.66, 44.73, 26.05, 25.29, 24.13, 24.07, 16.23, 16.18.
Embodiment 2
[0048] Slowly added triethyl phosphite (90.18g, 542.73mmol) into 3-chloro-1-(N,N-dimethyl)propylamine (60g, 493.38mmol), heated to 140°C, and stirred for 8h. After the reaction solution was cooled, it was quenched by adding water, extracted three times with ethyl acetate, and the organic phase was concentrated to dryness under reduced pressure to obtain a crude product.
[0049] Dissolve the above crude product in ethyl acetate, pass through hydrogen chloride, stir and crystallize at room temperature for 5 hours, suction filter and dry to obtain 3-(N,N-dimethyl)propyl phosphate diethyl ester hydrochloride ( The amount of compound 4) was 110.8 g, and the yield was 86.47%.
[0050] 1 H NMR (400MHz,D 2 O)δ3.88(tt, J=15.3,6.7Hz,1H),3.77–3.69(m,4H),3.01(q,J=7.0,6.2Hz,2H),2.60(s,6H),1.93( dq,J=12.0,6.4Hz,2H),1.66(dq,J=12.0,6.4Hz,2H),0.99(t,J=7.1Hz,6H). 13 C NMR (125MHz, D 2 O) δ61.30, 61.25, 57.74, 57.66, 44.73, 26.05, 25.29, 24.13, 24.07, 16.23, 16.18.
Embodiment 3
[0052] Slowly added triethyl phosphite (90.18g, 542.73mmol) into 3-chloro-1-(N,N-dimethyl)propylamine (60g, 493.38mmol), heated to 140°C, and stirred for 8h. After the reaction solution was cooled, it was quenched by adding water, extracted three times with ethyl acetate, and the organic phase was concentrated to dryness under reduced pressure to obtain a crude product.
[0053] Dissolve the above crude product in ethyl acetate, add concentrated hydrochloric acid (12N) dropwise to adjust the pH to 1-2, stir and crystallize at room temperature for 5 hours, filter with suction, and dry to obtain 3-(N,N-dimethyl) The amount of diethyl propyl phosphate hydrochloride (compound 4) was 109.8 g, and the yield was 85.69%.
[0054] 1 H NMR (400MHz,D 2 O)δ3.88(tt, J=15.3,6.7Hz,1H),3.77–3.69(m,4H),3.01(q,J=7.0,6.2Hz,2H),2.60(s,6H),1.93( dq,J=12.0,6.4Hz,2H),1.66(dq,J=12.0,6.4Hz,2H),0.99(t,J=7.1Hz,6H). 13 C NMR (125MHz, D 2 O) δ61.30, 61.25, 57.74, 57.66, 44.73, 26.05, 25.29, 24.13, 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com