Detection method of myocardial cell M3 receptor
A technology of cardiomyocytes and detection methods, applied in the field of cardiomyocyte M3 receptors, can solve the problems of cumbersome detection methods of cardiomyocytes M3 receptors, expensive detection reagents or instruments, and long time-consuming, so as to achieve easy routine and large-scale use , low cost, and the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
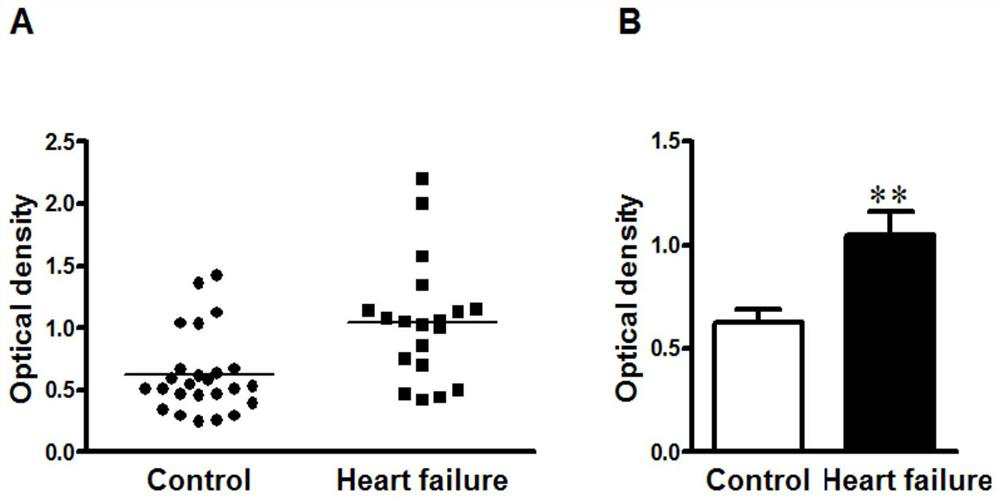
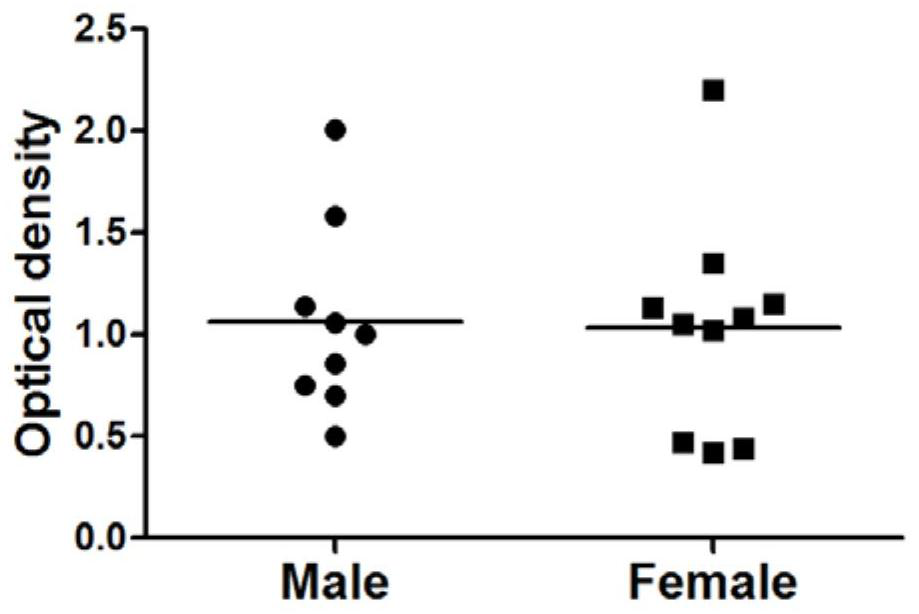
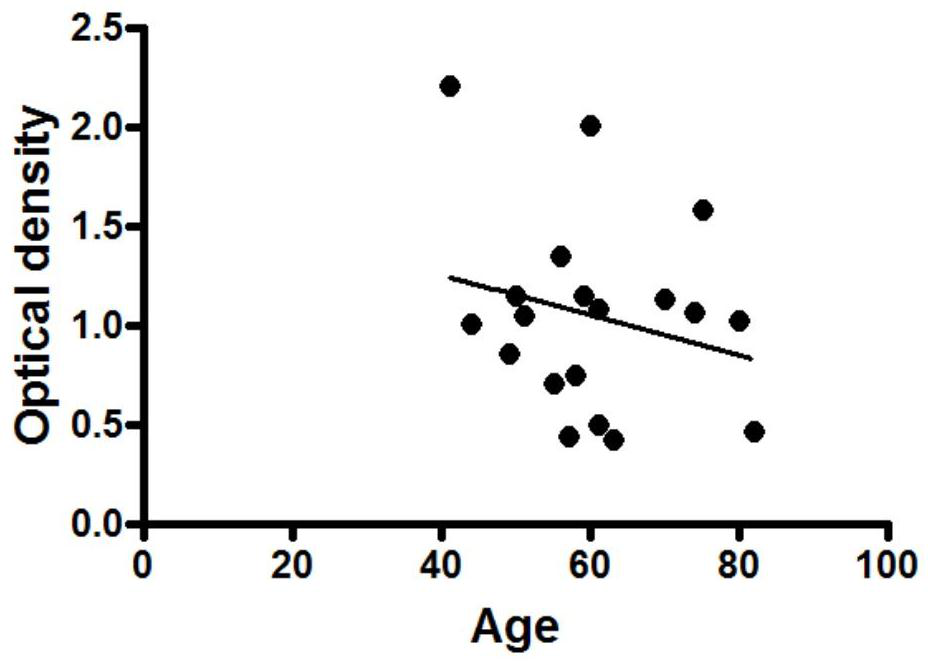
[0032] Specific embodiment one: the present invention provides a kind of detection method of cardiomyocyte M3 receptor, comprises the following steps:
[0033] S1 peptide synthesis: take 5-10mL samples to synthesize peptides according to the second loop functional epitope peptide sequence of the cardiomyocyte M3 receptor extracellular peptide sequence, the second loop functional epitope peptide sequence is CLFWQYFVGKRTVPPGEC at positions 205-220, Purify the synthesized peptide to 93%-98% by reverse-phase high-performance liquid chromatography to obtain a purified sample;
[0034] S2 ELISA plate coating: choose 50mM, pH9.6 carbonate coating buffer to dilute the purified sample to obtain 10μg·mL -1 The diluted sample solution, inject 100 μL of the diluted sample solution into each well of the microtiter plate for coating, and place it at 4°C for 12h-18h;
[0035] S3 blocking: Remove the coating solution from the coated wells of the microtiter plate, wash with PBST 4-6 times, 3 ...
specific Embodiment approach 3
[0044] Embodiment 3: The enzyme plate described in S2 is a 96-well medium-binding enzyme plate. The selection of medium-binding enzyme plate has the characteristics of high detection specificity and sensitivity, and low blank hole value. The other combinations and connections of this embodiment are the same as those of the second embodiment.
[0045] Embodiment 4: The diluted horseradish peroxidase-labeled goat anti-human IgG solution described in S4 is obtained by diluting horseradish peroxidase-labeled goat anti-human IgG with water at a mass ratio of 1:1000. The other combinations and connections of this embodiment are the same as those of the third embodiment.
[0046] Embodiment 5: The chromogenic agent described in S5 is a TMB substrate chromogenic agent or an OPD substrate chromogenic agent. The TMB substrate chromogenic reagent has high stability and lower sensitivity than the OPD substrate chromogenic reagent, and the OPD substrate chromogenic reagent has high sensi...
specific Embodiment approach 7
[0048] Embodiment 7: When the chromogenic agent is a TMB substrate chromogenic agent, use a microplate reader A490 to measure in S6; when the chromogenic agent is an OPD substrate chromogenic agent, use a microplate reader A450 to measure in S6. The other combinations and connections of this embodiment are the same as those of Embodiment 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com