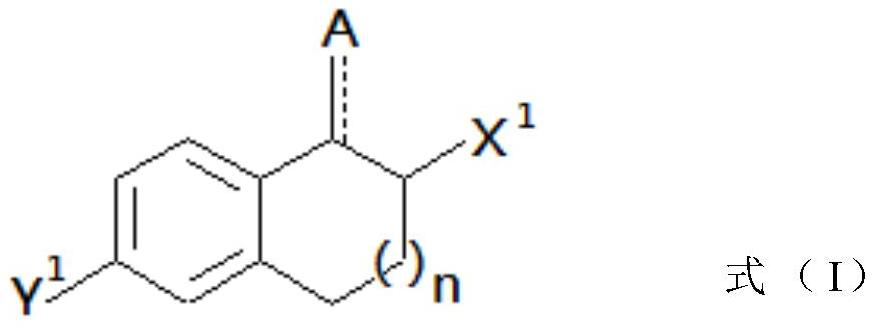
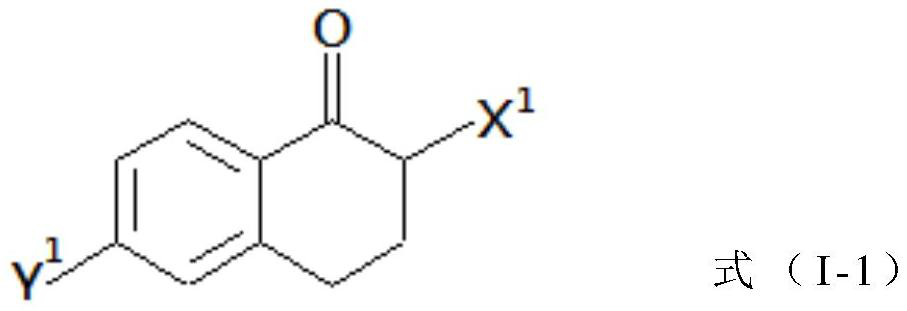
Peripheral alkyl and alkenyl chains extended benzene derivatives and pharmaceutical composition including same
A pharmaceutical and alkyl technology, applied in the field of phenyl derivatives with side chain alkyl and alkenyl extensions and pharmaceutical compositions including them, can solve the problem that dual inhibitors are rarely reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example I
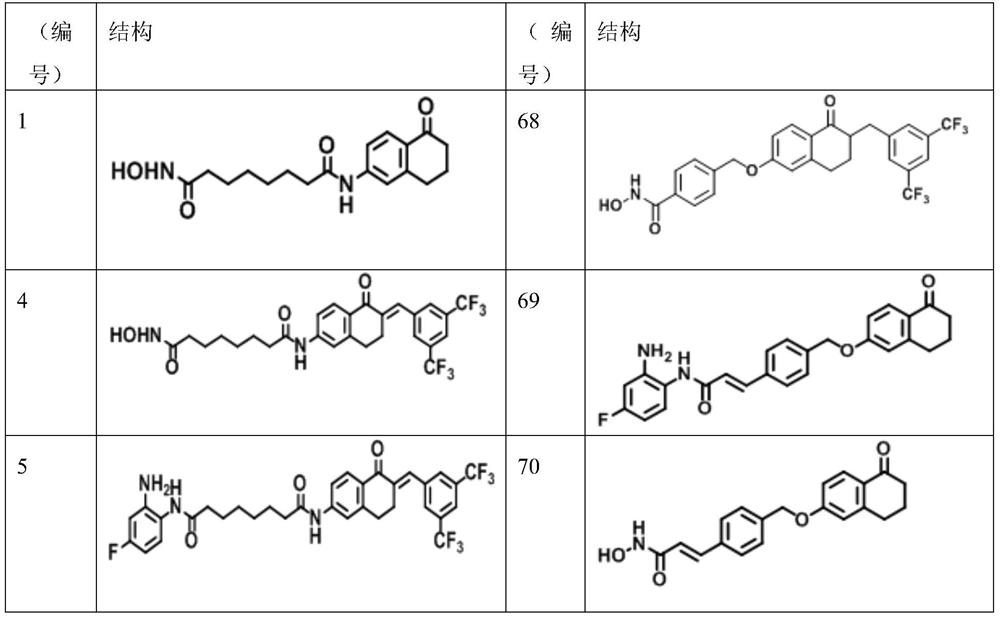
[0083] Example 1: Synthesis of Compound 1 to Compound 83
[0084] To demonstrate that compounds of the present disclosure can be appropriately synthesized and utilized, the synthetic steps of Compounds 1 to 83 depicted in Tables A to F are described in detail below.
[0085] Synthesis of Compound 1 to Compound 3
[0086] Process 01
[0087]
[0088] To compound 1a (6-amino-1-tetralone) (0.50 gram, 3.10 mmol), monomethyl suberate (0.70 gram, 3.72 mmol) and hydroxybenzotriazole at 0 ℃ (HOBt; 0.21 g, 1.55 mmol) in dichloromethane (DCM; 50 mL) were added N,N-diisopropylethylamine (DIPEA; 1.20 g, 9.31 mmol) and 1-ethyl - 3-(3-Dimethylaminopropyl)carbodiimide (EDCI; 0.82 g, 4.65 mmol). After the addition, the reaction mixture was allowed to warm slowly to room temperature (RT) and stirred overnight.
[0089] After the reaction was completed, the solvent was removed under reduced pressure. The residue was diluted with EtOAc and washed with saturated (sat) NH 4 Cl and saturat...
example II
[1232] Example II: Autotaxin / HDAC Inhibition Assay
[1233] In order to confirm that Compound 1 to Compound 83 of the present disclosure have the effect of inhibiting the activity of autotaxin and HDAC (dual ATX / HDAC inhibitor), the following experiments were carried out for evaluation.
[1234] Autotaxin Inhibition Screening Assay
[1235] ATX (autotaxin) activity was measured by choline release from LPC with or without the addition of test compounds (compound 1 to compound 83; concentrations of 4 micromolar, 20 micromolar and 50 micromolar). Mix twenty (20) Nanogram ATX (10803, Cayman, MI, USA) with 100 micromolar 14:0LPC (855575P, Avanti, Alabama, USA) (AL, USA)) were incubated together in a final volume of 100 μl buffer containing: 50 mM Tris pH 8.0, 0.01% Triton X-100, 50 mM CaCl 2 , 1 unit / ml choline oxidase, 2 units / ml horseradish peroxidase (HRP), 2 mmol homovanillic acid (homovanilic acid) (HVA). The relative amount of choline released was measured by HVA fluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com