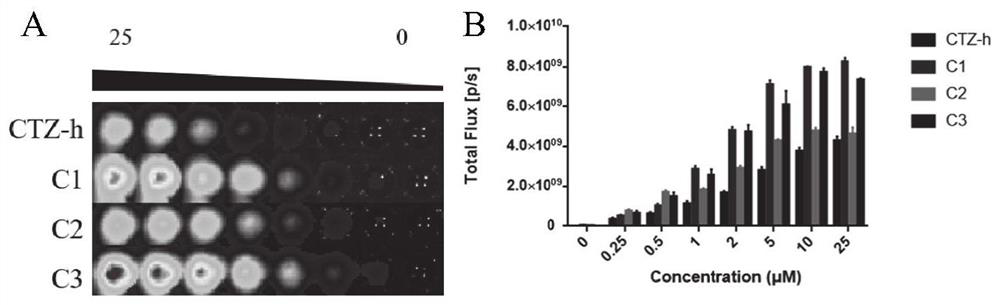
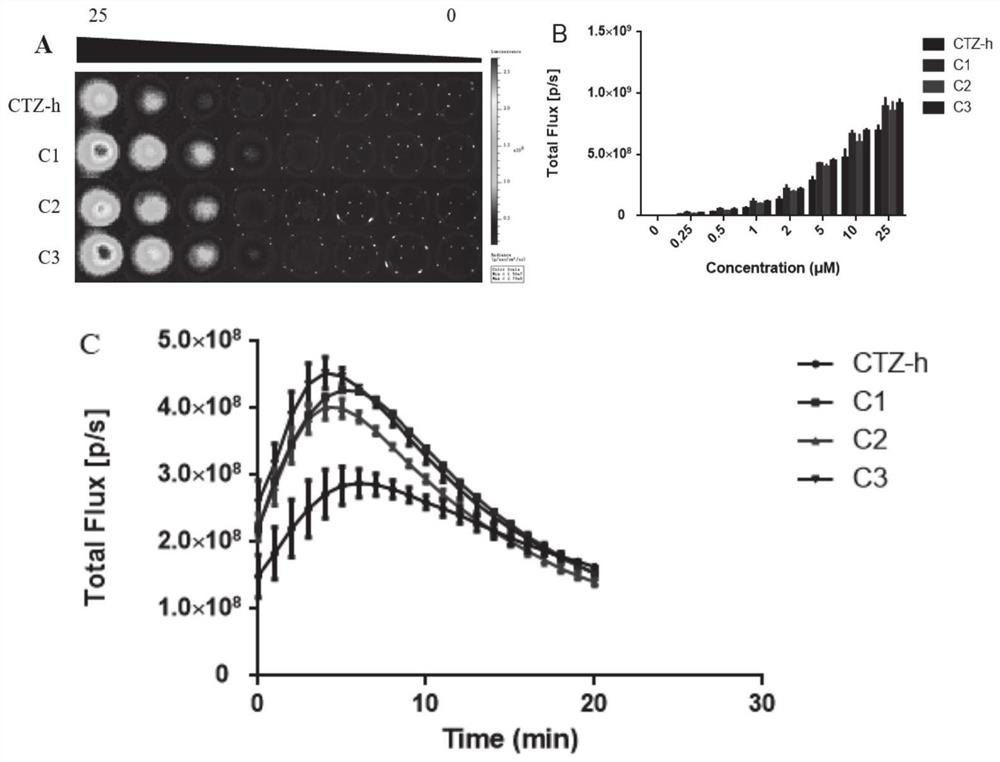
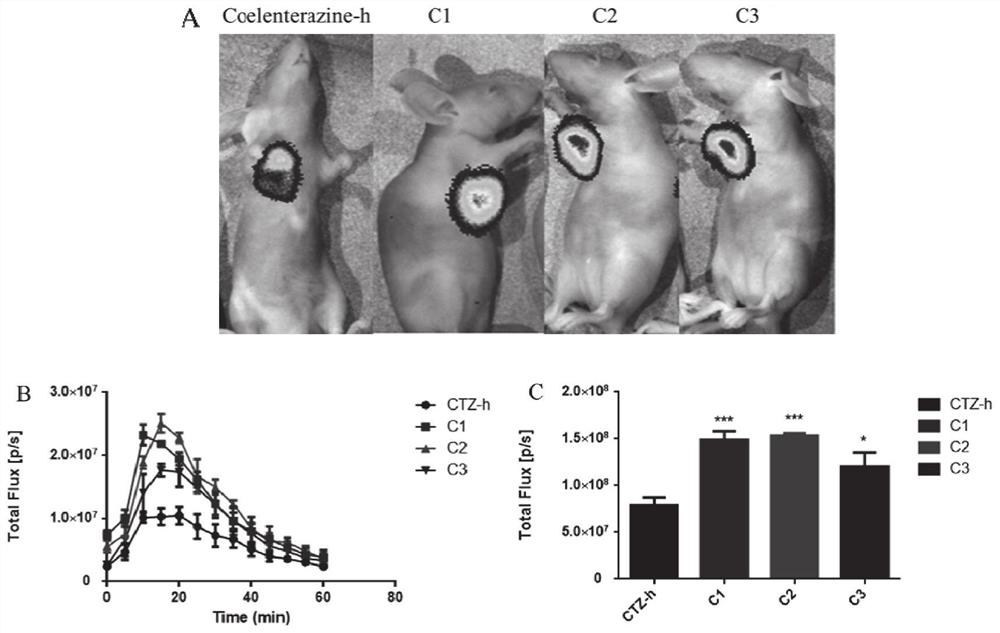
Coelenterazine-h-based deuterated compound as well as preparation method and application thereof
A technology of deuterated compounds and coelenterazine, applied in the field of deuterated compounds based on coelenterazine-h and preparation thereof, can solve the problems of short bioluminescence emission wavelength, low bioluminescence intensity, short duration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: 2-(1,1-dideuterium-1-phenylmethyl)-5-deuterium-6-(4-hydroxyphenyl)-8-(1,1-dideuterium-1-(2 , Preparation of 3,4,5,6-pentadeuteriophenyl)methyl)imidazol[1,2-a]pyrazin-3(7H)-one (C1).
[0067] Preparation of 1,1-dideuterium-1-(2,3,4,5,6-pentadeuteriophenyl)-1-bromomethane:
[0068] Deuterated toluene (1g, 9.98mmol, toluene-d 8 ) and N-bromosuccinimide (1.78g, 9.98mmol) were dissolved in 50mL of carbon tetrachloride, and then tert-butyl peroxybenzoate (581mg, 2.99mmol) was added. React at 90°C for 2 hours, use TLC to monitor the reaction, the reaction is complete, stop the reaction, filter, remove the succinimide in the reaction solution, collect the filtrate, spin dry, add 100-200 mesh silica gel to mix the sample, and mix with 200 -300 mesh silica gel column, with petroleum ether as eluent, to obtain intermediate compound 1, R f About 0.5, developer: petroleum ether, 1.1 g of colorless oily compound, yield 62%. 13 C NMR(101MHz,DMSO)δ138.22,129.56,129.32,12...
Embodiment 2
[0080] Example 2: 2-benzyl-6-(4-hydroxyphenyl)-8-((2,3,4,5,6-pentadeuteriophenyl)methyl)imidazo[1,2-a]pyridine Preparation of azin-3(7H)-ones (C2).
[0081] 2-benzyl-6-(4-hydroxyphenyl)-8-((2,3,4,5,6-pentadeuteriophenyl)methyl)imidazo[1,2-a]pyrazine-3( Preparation of 7H)-ketone:
[0082] 2-amino-3-(1,1-dideuterium-1-(2,3,4,5,6-pentadeuteriophenyl)methyl)-5-(4-hydroxyphenyl) prepared in Example 1 -pyrazine (100mg, 0.35mmol) and 1,1-diethoxy-3-phenylpropan-2-one (156mg, 0.73mmol) prepared in Example 1 were dissolved in 10mL of dehydrated ethanol, under nitrogen Under protection, after stirring at room temperature for 2 hours, concentrated hydrochloric acid (366mg, 3.5mmol) was added, the reaction solution was transferred to 80°C, reacted for 8 hours, cooled to room temperature, spin-dried, and thin-layer chromatography was performed. : Methanol = 15: 1 as the chromatographic solution, the target color band is obtained, and the target color band is washed with dichloromethane:...
Embodiment 3
[0085] Example 3: 5-deuterium-6-(4-hydroxyphenyl)-2,8-bis(1,1-dideuterium-1-phenylmethyl)imidazo[1,2-a]pyrazine-3 Preparation of (7H)-ketones (C3).
[0086] Preparation of 2-amino-3-benzyl-5-bromo-pyrazine:
[0087] Activated zinc powder (3.06g, 46.77mmol) was added to a 50mL double-necked flask, and under nitrogen protection at room temperature, a solution of iodine element (356mg, 1.4mmol) was added (iodine element was dissolved in ultra-dry tetrahydrofuran and ultra-dry N,N -Dimethylformamide, ultra-dry tetrahydrofuran:N,N-dimethylformamide=5:1), stirring, the color of the iodine element fades. Benzyl bromide (4 g, 23.39 mmol) was added to the above solution using a syringe and refluxed at 85°C. After 3 h, the reaction solution (uniform gray solution) was cooled to room temperature. Dissolve 2-amino-3,5-dibromopyrazine (3.94 g, 15.59 mmol) and bistriphenylphosphine palladium dichloride (328 mg, 467.67 μmol) in ultra-dry N,N-dimethylformamide solution , which was added d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com