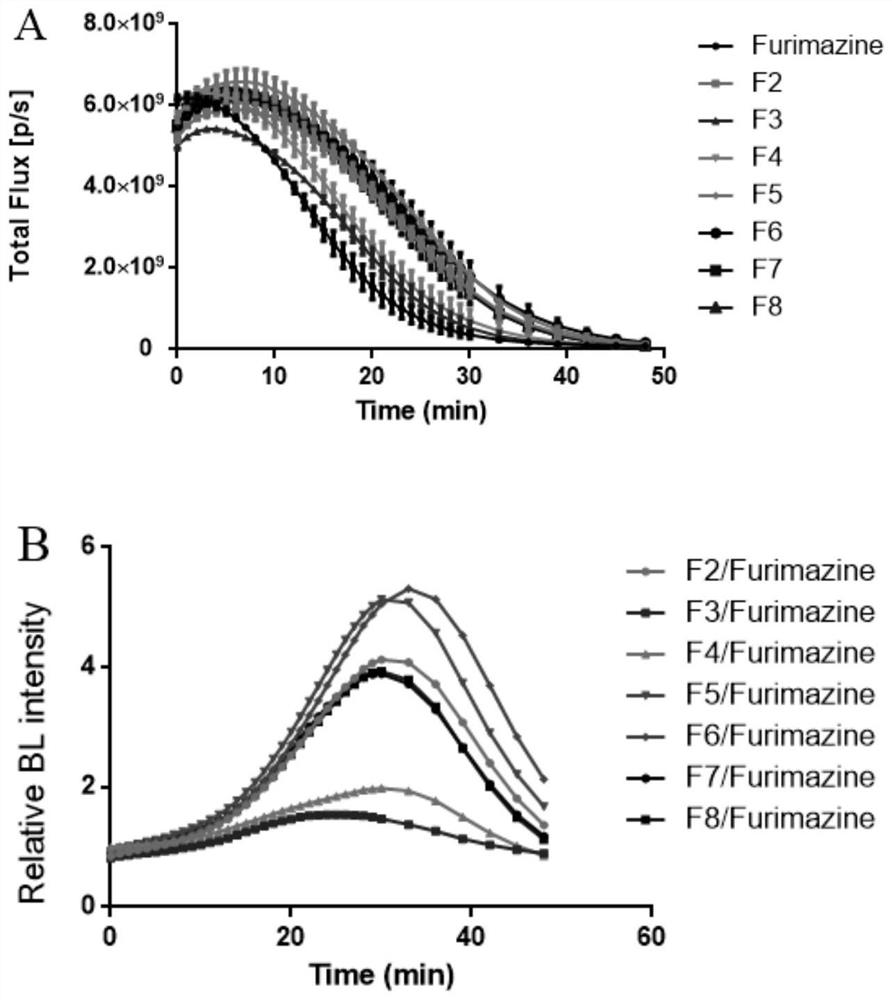
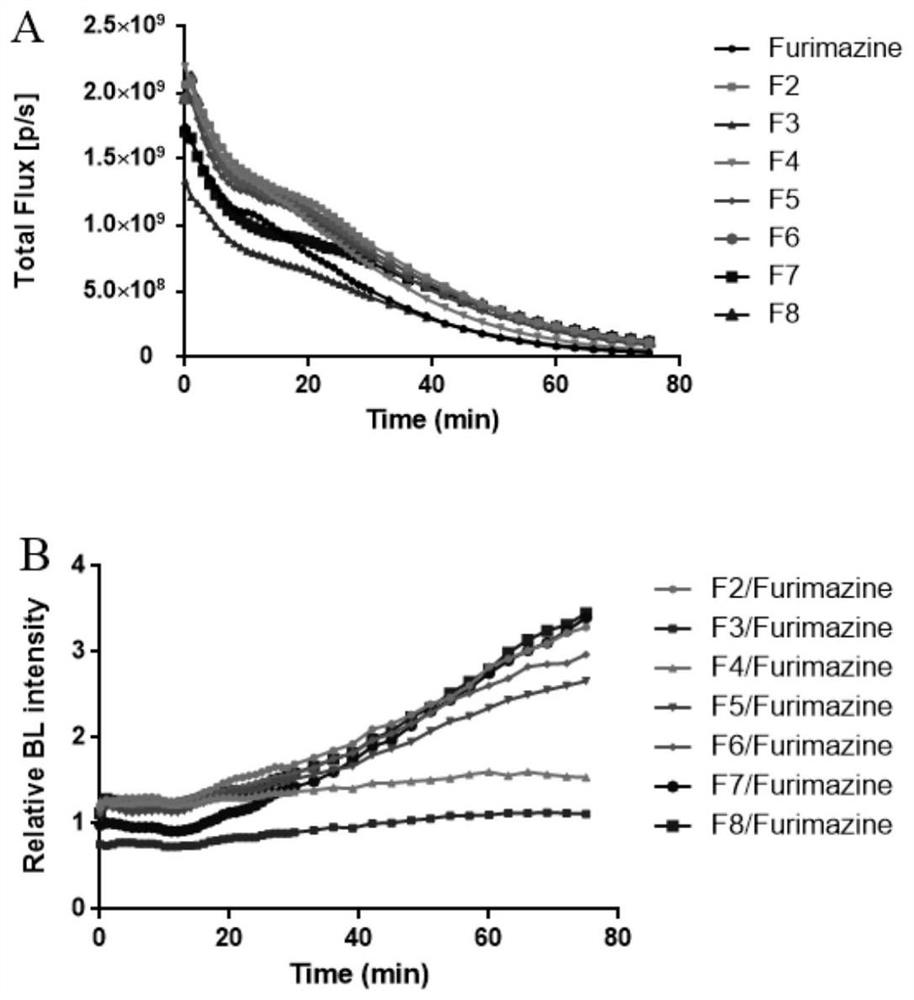
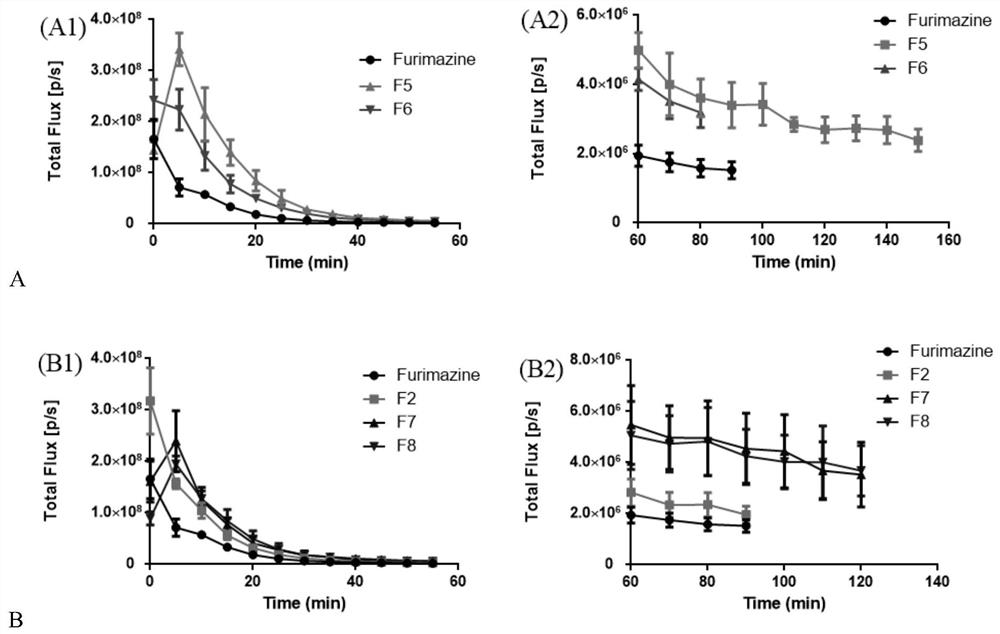
Imidazopyrazinone compound as well as preparation method and application thereof
A technology of imidazo and compound, which is applied in the field of imidazopyrazinone compounds and their preparation, can solve the problems of fast decay speed, low bioluminescence intensity, and Millet's constant, and achieve the effect of increasing half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 : 2-(furan-2-ylmethyl)-6-phenyl-8-((1-deuterium-1-phenyl)methyl)imidazo[1,2-a]pyrazine-3(7H) - Preparation of ketone (F2)
[0075] Preparation of 1-deuterium-1-phenylcarbinol (2) (phenylmethan-d-ol):
[0076] Deuterated lithium aluminum hydride (160mg, 3.82mmol) was added to a 25mL double-neck flask, under nitrogen protection, 5mL of ultra-dry tetrahydrofuran was added, in an ice bath, at 0°C, using a syringe to drop Benzaldehyde (540mg, 5.10mmol) in ultra-dry tetrahydrofuran, reacted at 0°C for 30min, monitored by TLC, added 160μL of water, 160μL of 15% sodium hydroxide, and 480μL of water, continued to stir for 5min, and filtered through diatomaceous earth , diatomite was washed with diethyl ether, the filtrate was collected, washed with saturated brine to obtain the organic phase compound, dried over anhydrous sodium sulfate, mixed with 100-200 mesh silica gel, packed into a column with 200-300 mesh silica gel, and prepared with a certain proportion of p...
Embodiment 2
[0097] Example 2 : 2-(furan-2-ylmethyl)-6-phenyl-8-((1,1-dideutero-1-phenyl)methyl)imidazo[1,2-a]pyrazine-3 Preparation of (7H)-ketone
[0098] 1,1-Dideuterium-1-phenylmethanol (4) (phenylmethan-d 2 -ol) preparation:
[0099] Deuterated lithium aluminum hydride (201mg, 4.79mmol) was added to a 25mL double-neck flask, and under nitrogen protection, 5mL of ultra-dry tetrahydrofuran was added, and in an ice bath, at 0°C, using a syringe, dropwise added Ethyl benzoate (600mg, 4.00mmol), react at room temperature for 1h, monitor the reaction by TLC, cool the reaction solution to 0°C, add 0.5mL of heavy water, and continue stirring for 30min at 0°C, using diatom Filter with soil, wash with ether, dry with anhydrous sodium sulfate, spin dry, mix the sample with 100-200 mesh silica gel, pack with 200-300 mesh silica gel, and use a certain proportion of ethyl acetate and petroleum ether for gradient elution to obtain Intermediate compound 4, 380mg of colorless transparent oily com...
Embodiment 3
[0114] Example 3 : 2-(furan-2-ylmethyl)-6-phenyl-8-((1,1-dideuterio-1-(2,3,4,5,6-pentadeuteriophenyl))methanol base) imidazo[1,2-a]pyrazin-3(2H)-one
[0115] 1,1-Dideuterium-1-(2,3,4,5,6-pentadeuteriophenyl)-1-bromomethane (1)(1-(bromomethyl-d 2 )benzene-2,3,4,5,6-d 5 ) preparation:
[0116] Deuterated toluene (1g, 9.98mmol, toluene-d 8 ) and N-bromosuccinimide (1.78g, 9.98mmol) were dissolved in 50mL of carbon tetrachloride, and then tert-butyl peroxybenzoate (581mg, 2.99mmol) was added. React at 90°C for 2 hours, use TLC to monitor the reaction, the reaction is complete, stop the reaction, filter, remove the succinimide in the reaction solution, collect the filtrate, spin dry, add 100-200 mesh silica gel to mix the sample, and mix with 200 -300 mesh silica gel column, with petroleum ether as eluent, to obtain intermediate compound 1, R f About 0.5, developer: petroleum ether, 1.1 g of colorless oily compound, yield 62%. 13 C NMR(101MHz,DMSO)δ138.22,129.56,129.32,129....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com