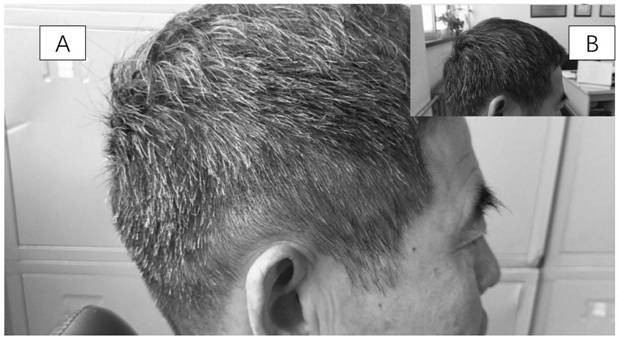
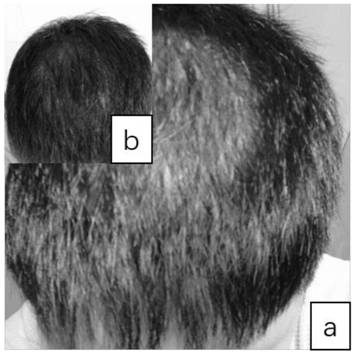
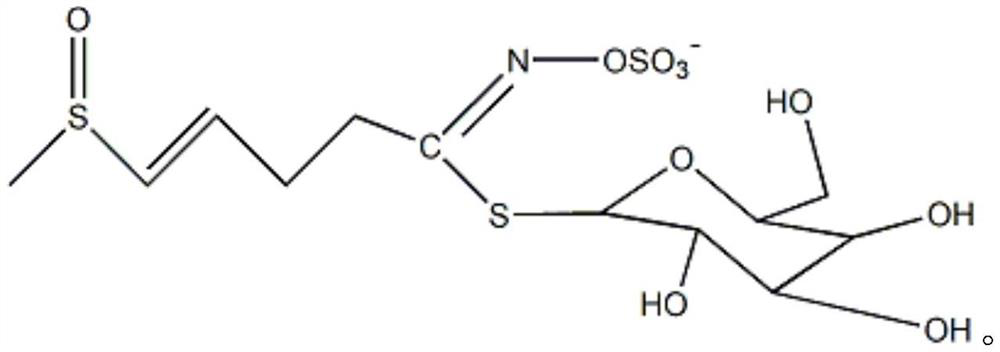
Pharmaceutical composition for improving and treating leukotrichia and/or alopecia and preparation method thereof
A composition and drug technology, applied in the pharmaceutical composition for improving and treating gray hair and/or hair loss, the preparation field of the pharmaceutical composition can solve side effects, unreported glucosinolate biological activity, poor effect, etc. problems, to promote hair growth, improve and treat gray hair and/or hair loss, and stop progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054]
[0055] A. Crushing the raw materials
[0056] Using a pulverizer to pulverize one or more of the seeds, flowers, stems and leaves of cruciferous plants as raw materials, and collect the pulverized matter.
[0057] B. Extraction of glucoraphanin
[0058] After heating the deionized water to boiling, add the pulverized product obtained in step A according to the solid-to-liquid ratio of 1:5 to 1:50, extract with boiling water for 10 to 30 minutes, and filter with filter cloth to obtain the water extract. Repeat the extraction once, and collect the obtained glucoraphanin water extract.
[0059] Determine the amount of activated carbon according to the mass ratio of activated carbon to glucoraphanin water extract of 10:1 to 10:5, add the water extract to activated carbon, stir and adsorb at a temperature of 0 to 50°C for 10 to 60 minutes, and filter An activated carbon filter cake adsorbed with glucoraphanin is obtained. The activated carbon filter cake is mixed with...
Embodiment 1
[0097] Example 1 Preparation of Granule 1 Containing Glucoraphanin and Myrosinase
[0098] Take 200g of the glucoraphanin extract prepared by the above method I. (the purity of glucoraphanin is 95%) and 19.8kg of dextrin are mixed and dissolved in 100L of deionized water, and then spray-dried. The air inlet temperature of the spray drying was set to 180°C, the outlet air temperature was adjusted to 80°C, the liquid inlet speed was 5L / h, the product was collected, and the glucoraphanin spray-dried powder was prepared. Take 200mg of the myrosinase extract prepared by the above-mentioned method II. and 19.8kg of dextrin are mixed and dissolved in 100L of deionized water, and then spray-dried. The air inlet temperature of spray drying was set to 180°C, the outlet air temperature was adjusted to 80°C, and the liquid inlet speed was 5L / h. The product was collected to prepare myrosinase spray-dried powder. The glucoraphanin spray-dried powder and the myrosinase spray-dried powder ...
Embodiment 2
[0099] Example 2 Preparation of Granule 2 Containing Glucoraphanin and Myrosinase
[0100] Take 300g of the glucoraphanin extract prepared according to the above method I. (the purity of glucoraphanin is 92%) and 4.7kg of dextrin are mixed and dissolved in 2.5L of deionized water, and then spray-dried. The air inlet temperature of the spray drying was set to 180°C, the outlet air temperature was adjusted to 80°C, the liquid inlet speed was 5L / h, the product was collected, and the glucoraphanin spray-dried powder was prepared. Take 300 mg of the myrosinase extract prepared by the above method II. and 4.7 kg of dextrin, mix and dissolve in 2.5 L of deionized water, and then spray dry. The air inlet temperature of spray drying was set to 180°C, the outlet air temperature was adjusted to 80°C, and the liquid inlet speed was 5L / h. The product was collected to prepare myrosinase spray-dried powder. The spray-dried powder of glucoraphanin and the spray-dried powder of myrosinase w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com