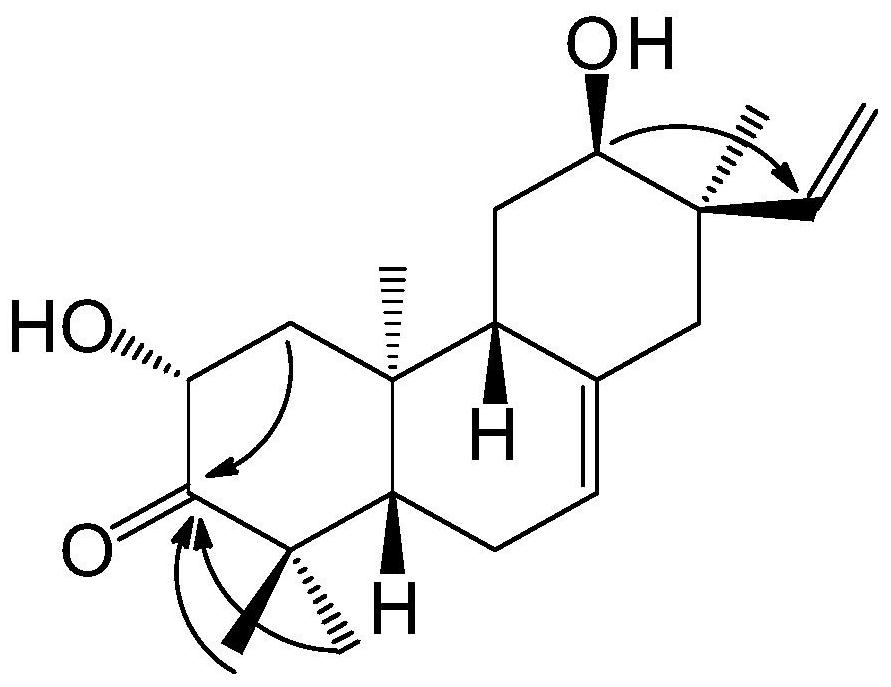
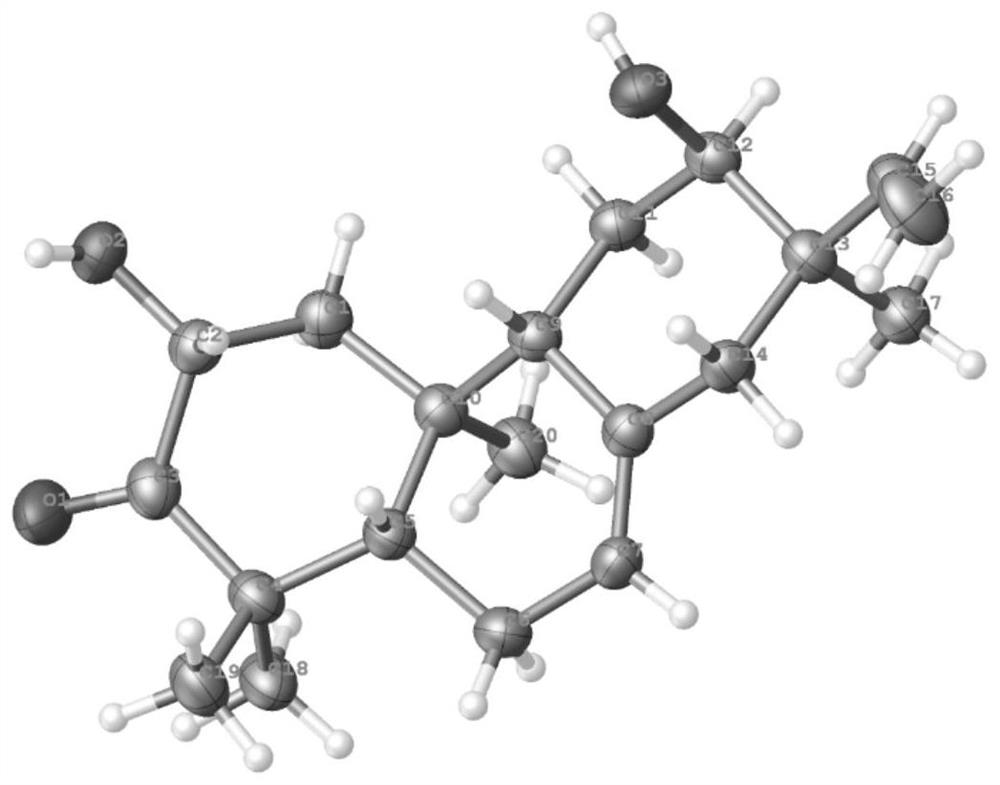
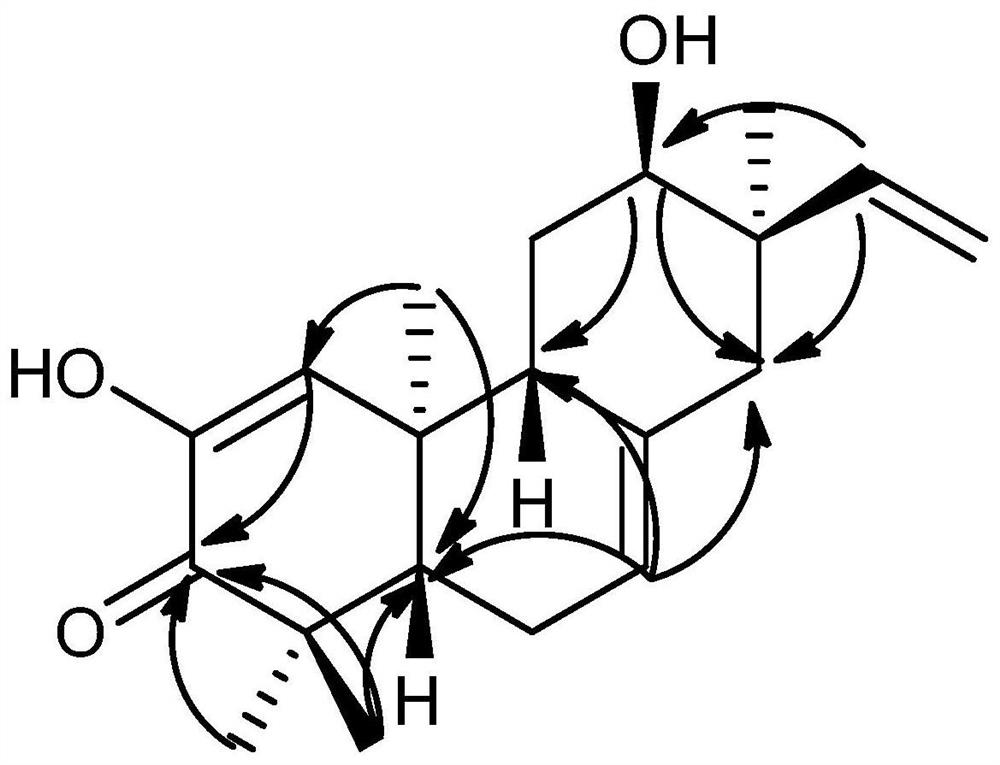
Tricyclic diterpenoid compound with antitumor activity, and preparation method and application thereof
A technology with anti-tumor activity and tricyclic diterpene, which is applied in the field of medicine, can solve the problems of high cost, high toxicity and side effects, etc., and achieve the effect of low cost, strong anti-tumor activity, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation method of the tricyclic diterpenoid compound with antitumor activity is characterized in that it comprises the steps of:
[0046] (1) Extraction:
[0047] Take 10 kg of Euphorbia euphorbia medicinal material, add 10 times the volume concentration of 95% ethanol to reflux and extract twice, each time for 2 hours, to obtain an ethanol extract.
[0048] (2) Extraction
[0049] Concentrate the ethanol extract from step (1) until there is no alcohol smell, add 3.0L of water to suspend, add an equal volume of ethyl acetate to extract 3 times, combine the ethyl acetate layers, and concentrate under reduced pressure at 45°C to obtain the extract;
[0050] (3) Separation
[0051] Take the extract from step (2), separate it by medium-pressure silica gel column chromatography, and use petroleum ether-ethyl acetate gradient elution with a volume ratio of 100:0-0:100 to obtain fractions JDJ-HeN-EA-1-14;
[0052] Fraction JDJ-HeN-EA-9 was separated by Sephadex LH-2...
Embodiment 2
[0075] Embodiment 2 in vitro antitumor test
[0076] 1. Experimental materials
[0077] RPMI1640 (Biological Industries, article number: 01-100-1ACS), IMDM (Biological Industries, article number: 01-058-1ACS), DMEM (Biological Industries, article number: 06-1055-57-1ACS), Fetal Bovine Serum (Biosera, article number ; FB-1058 / 500), CCK8 (Caosu, product number: C0005), trypsin EDTA solution 0.25% (Yuanpei, product number: S310KJ), DMSO (Sinopharm, product number: 30072418), Staurosporine (Caosu, product number: T6680 ), paclitaxel (Dingke Medical), 384-well cell culture plate (BIOFIL, catalog number: TCP-011-384), EnVision, PerkinElmer
[0078] 2. Experimental method
[0079] 2.1 Cells and passage
[0080] Chronic myeloid leukemia K562 (cell culture medium, IMDM+10%FBS+1%P / S), histiocytic lymphoma U-937 (RPMI1640+10%FBS+1%P / S), colorectal cancer LOVO (DMEM +10% FBS +1% P / S).
[0081] Cell subculture: When the cells cover 80-90% of the culture dish, digest the cells with 0.25%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com