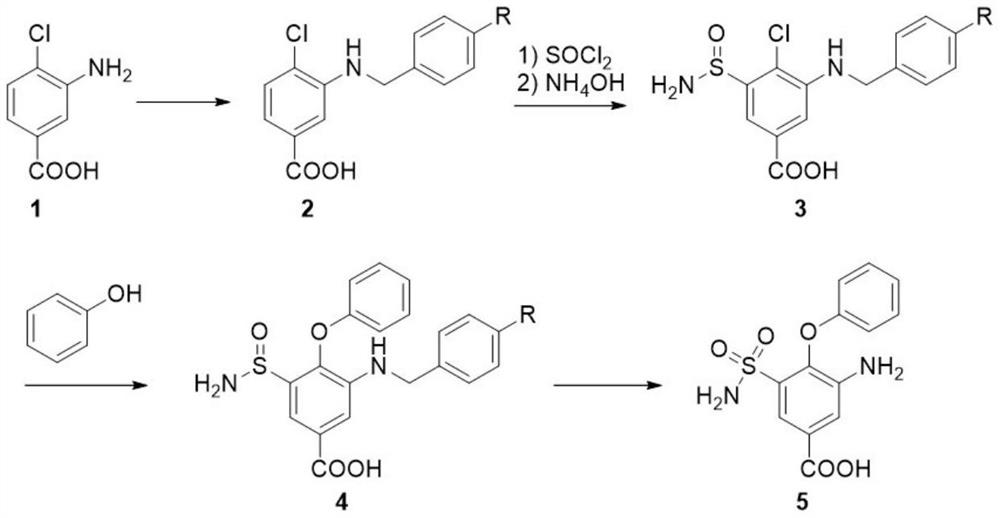
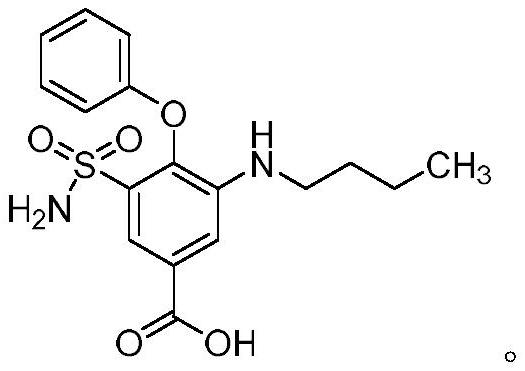
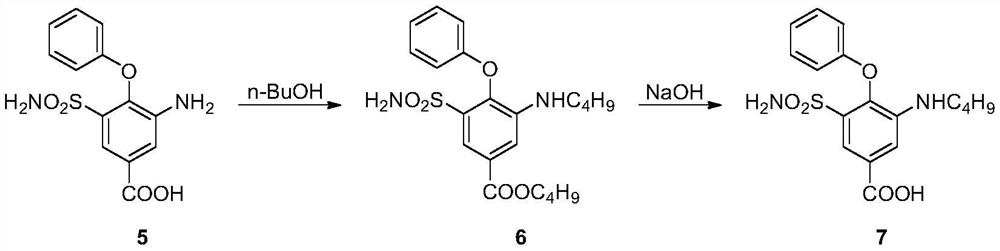
Synthesis method of intermediate of bumetanib
A synthetic method and intermediate technology, applied in the field of medicine, to achieve the effects of facilitating production organization, avoiding nitration reactions, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: preparation compound 2a
[0065]
[0066] 3-amino-4-chlorobenzoic acid (100g, 0.583mol) was added to the reaction flask, dichloromethane (600ml) was added to dissolve, triethylamine (162ml, 1.166mol) was added, benzyl chloride (81.18g, 0.641mol) was added dropwise, the drop rate was controlled, the temperature was not higher than 40°C, the dropwise addition was completed, and the reaction was monitored by TLC until the reaction was completed, and 0.5mol / L sodium hydroxide solution (500ml) was added for extraction, the aqueous phase was separated, and the solution was washed with dilute hydrochloric acid Adjust the pH value to 4-5, crystallize at 0-10°C to obtain an off-white solid, and blow dry at 40-50°C to obtain compound 2a (141.9g), with a yield of 93%.
[0067] ESI-MS(+):262.2[M+H] + . 1 H-NMR (400MHz, DMSO-d6+D 2 O): 7.49(d, 1H, J=7.0Hz), 7.47(d, 1H, J=7.0Hz), 7.31-7.27(m, 6H), 4.31(s, 2H).
Embodiment 2
[0068] Embodiment 2: preparation compound 2a
[0069]
[0070] 3-amino-4-chlorobenzoic acid (100g, 0.583mol) was added to the reaction flask, dichloromethane (600ml) was added to dissolve, triethylamine (162ml, 1.166mol) was added, benzyl bromide (109.64g, 0.641mol) was added dropwise, the drop rate was controlled, the temperature was not higher than 40°C, the dropwise addition was completed, and the reaction was monitored by TLC until the reaction was completed, and 0.5mol / L sodium hydroxide solution (500ml) was added for extraction, the aqueous phase was separated, and the solution was washed with dilute hydrochloric acid Adjust the pH value to 4-5, and crystallize at 0-10°C to obtain an off-white solid, which is air-dried at 40-50°C to obtain compound 2a (138.8g), with a yield of 91%.
Embodiment 3
[0071] Embodiment 3: preparation compound 2a
[0072] 3-amino-4-chlorobenzoic acid (100g, 0.583mol) was added to the reaction flask, dichloromethane (600ml) was added to dissolve, triethylamine (162ml, 1.166mol) was added, benzyl bromide (149.66g, 0.875mol) was added dropwise, the drop rate was controlled, and the temperature was not higher than 40°C. After the dropwise addition was completed, the reaction was monitored by TLC until the reaction was completed, and 0.5mol / L sodium hydroxide solution (500ml) was added for extraction, and the aqueous phase was separated and washed with dilute hydrochloric acid Adjust the pH value to 4-5, crystallize at 0-10°C to obtain an off-white solid, and blow dry at 40-50°C to obtain compound 2a (140.3g), with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com