Synthetic method of linaclotide
A synthesis method and linaclotide technology, applied in the field of linaclotide synthesis, can solve the problems of deterioration of production environment, increase of material cost, unfriendly environment, etc., to increase safety and environmental protection, and reduce the use of TFA , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
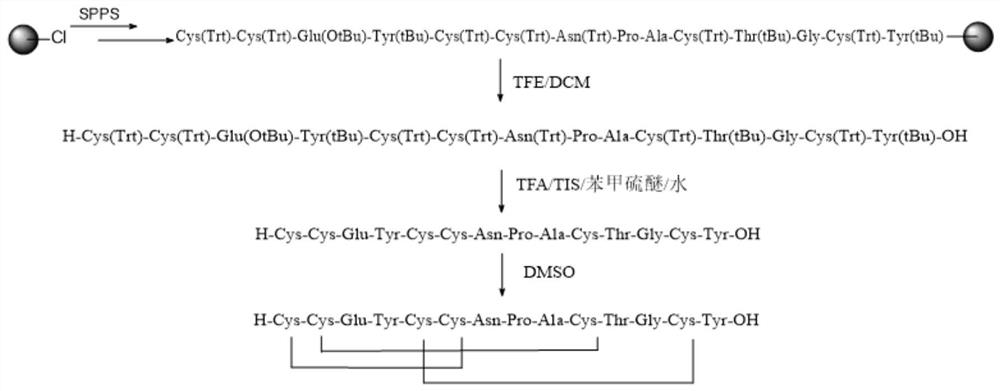
[0042] A method for synthesizing linaclotide of the present invention, comprising:
[0043] a) obtaining linaclotide peptide resin;
[0044] b) cleaving the linaclotide peptide resin through the first lysate and the second lysate in sequence to obtain a linear peptide;
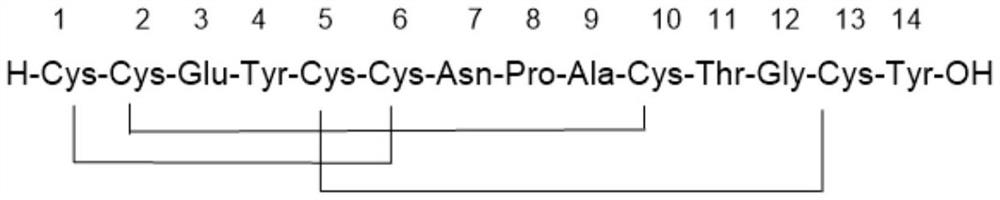
[0045] c) the linear peptide is cyclized to obtain linaclotide;
[0046] The first lysate comprises TFE and DCM;
[0047] The second lysate includes TFA, TIS, thioanisole and water.
[0048] In some embodiments, the synthesis method of linaclotide specifically includes the following steps:
[0049] 1) Preparation of amino acid resin: using solid-phase dichloro resin as a carrier, connecting Fmoc-Tyr(tBu)-OH under the catalysis of alkali to obtain Fmoc-Tyr(tBu)-2CTC resin;
[0050] 2) Preparation of peptide resin: use Fmoc-Tyr(tBu)-2CTC resin as a carrier, connect the protected amino acid, remove the amino acid protecting group, and then connect the protected amino acid to connect the amino group and the pr...
Embodiment 1
[0057] Embodiment 1: the preparation of the Fmoc-Tyr (tBu)-2CTC resin that substitution degree is 0.58mmol / g
[0058] Weigh 50g of 2CTC Resin with a substitution degree of 1.26mmol / g in a solid-phase reaction column, add DMF, bubble and swell with nitrogen for 30min, and drain the solvent; weigh Fmoc-Tyr(tBu)-OH (57.89g, 126mmol) DMF was dissolved, added to a solid-phase reaction column, and DIPEA (16.28g, 126mmol) was added. After reacting with nitrogen gas for 30min, additional DIPEA (8.14g, 63mmol) was added. After reacting for 1.5 hours, methanol was added to block for 1 hour, and washed with DCM three times. After methanol shrinkage, the resin was drained to obtain Fmoc-Tyr(tBu)-2CTC resin, and the detected substitution degree was 0.58mmol / g.
Embodiment 2
[0059] Embodiment 2: the preparation of the Fmoc-Tyr (tBu)-2CTC resin that substitution degree is 0.64mmol / g
[0060] Weigh 50g of 2CTC Resin with a substitution degree of 1.40mmol / g in a solid-phase reaction column, add DMF, bubble and swell with nitrogen for 30min, and drain the solvent; weigh Fmoc-Tyr(tBu)-OH (64.33g, 140mmol) DMF was dissolved, added to a solid-phase reaction column, and DIPEA (18.09g, 140mmol) was added. After reacting with nitrogen gas for 30min, additional DIPEA (9.04g, 70mmol) was added. After reacting for 1.5 hours, methanol () was added to block for 1 hour, and washed with DCM. Three times, the resin was dried after methanol shrinkage to obtain Fmoc-Tyr(tBu)-2CTC resin, and the detected substitution degree was 0.64mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com