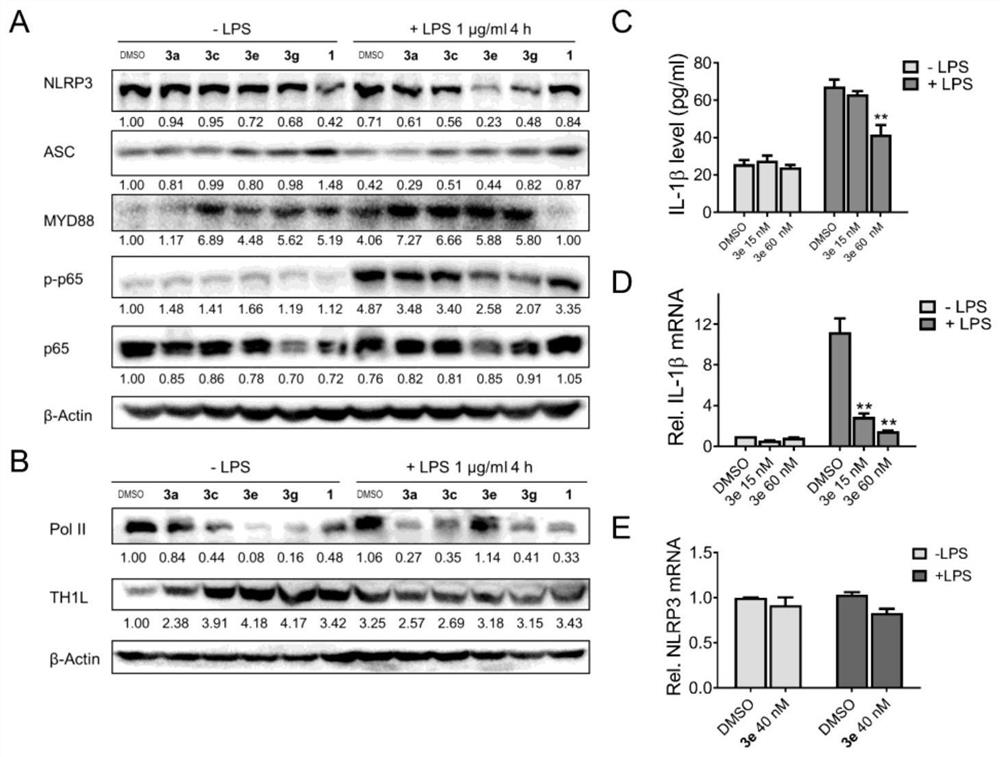
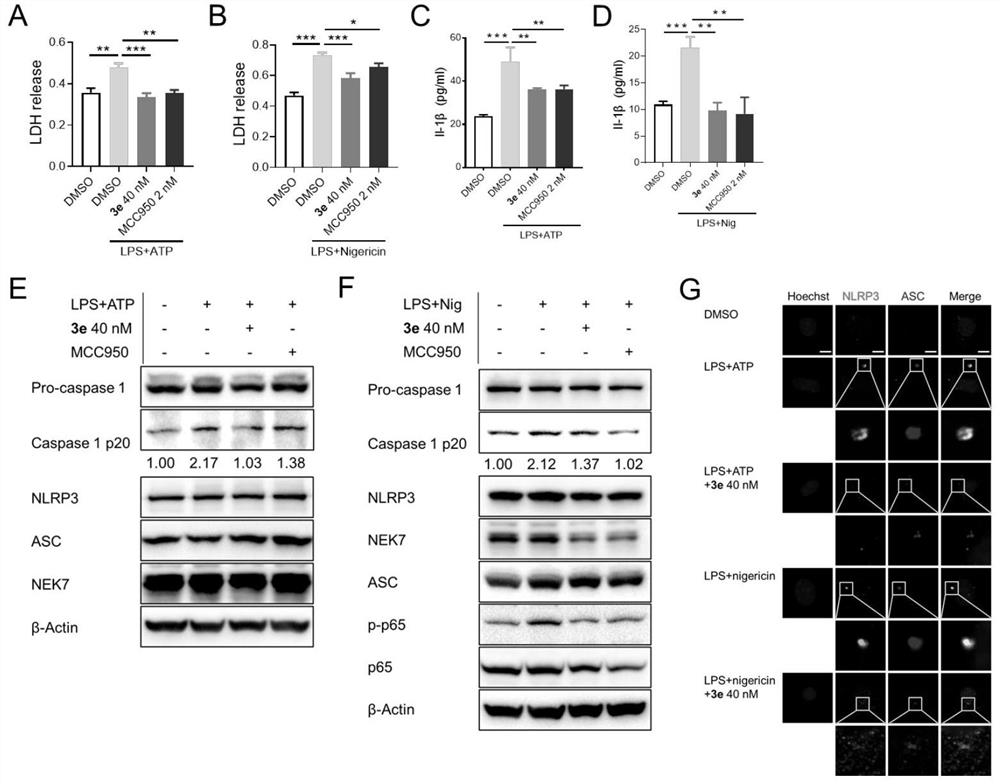
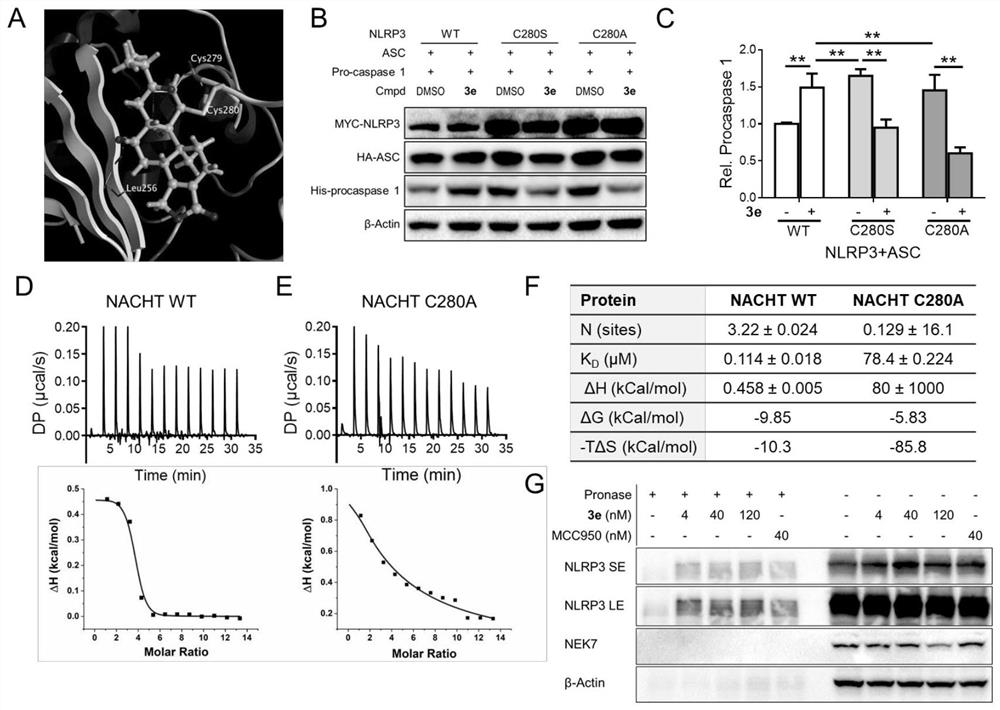
Application of tripdiolide in preparation of medicine for preventing and/or treating NLRP3 inflammasome-mediated inflammatory diseases
An inflammatory disease, triptolide technology, applied in the field of medicine, can solve the problems of liver toxicity, increased activity of liver function-related enzymes, and low activity of NLRP3 inhibitors in vivo, achieving a wide therapeutic window and reducing the level of verification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The present embodiment provides a kind of synthetic method of triptolide, specifically as follows:
[0054]
[0055] Dissolve triptolide (27mg, 0.07mmol) in anhydrous tetrahydrofuran (4mL), add LiBH successively 4 · THF solution (2N, 84 μL, 0.17 mmol) and BF 3 ·Et 2 O solution (45 μL, 0.35 mmol), stirred at room temperature for 3 h under the protection of argon. After the reaction was completed, dilute hydrochloric acid was added to quench the reaction and continued to stir for 10 min, and dichloromethane was added to extract three times. Column chromatography obtained 7 mg of triptolide white, with a yield of 25.9%.
[0056] The product is characterized by: 1H NMR (500MHz, CDCl3, δ): 4.73(m, 2H), 3.85(d, J=1.5Hz, 1H), 3.83(d, J=3.0Hz, 1H), 3.55(d, J =12.0Hz, 1H), 3.44(dd, J=3.0Hz, J=1.0Hz, 1H), 2.53(m, 2H), 2.29(m, 4H), 1.97(m, 1H), 1.63(m, 2H ), 1.28 (m, 2H), 1.23 (s, 3H), 1.03 (d, J=7.0Hz, 3H), 0.87 (d, J=7.0Hz, 3H).13C NMR (125MHz, CDCl3, δ): 173.9, 162.1, ...
Embodiment 2
[0057] Embodiment 2 biological experiment
[0058] 1. Method
[0059] 1. Animals and reagents
[0060] All animal experiments were approved by the ethics review of the University of Macau. C57BL / 6J mice were provided by the Specific Pathogen Free (SPF) Animal Center of the University of Macau. One week before the experiment, the mice should acclimate to the environment in a 12-h dark / light cycle, with free access to food and water, and the temperature should be controlled between 22.5±0.5°C. Tumor necrosis factor-α (TNF-α) was purchased from PeproTech. Lipopolysaccharide (LPS) and Gram-positive bacteria Bacillus subtilis flagellin (FLA-BS) were purchased from InvivoGen. MCC950 was purchased from Cayman Chemical. All other materials and reagents were purchased from Thermo Fisher Scientific and Sigma-Aldrich or otherwise indicated.
[0061] 2. Cell culture
[0062] AD-293 cell line was purchased from Strategene (La Jolla, CA, USA). AD-293 cells were maintained in DMED hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com