Application of leucyl-tRNA synthetase 2
A technology of synthase and leucyl, which is applied to medical preparations, enzymes, ligases and other directions containing active ingredients, can solve the problems of premature ovarian failure, lack of research on protein function and mechanism, and inhibition of yeast mitochondrial function, etc. The effect of promoting post-repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
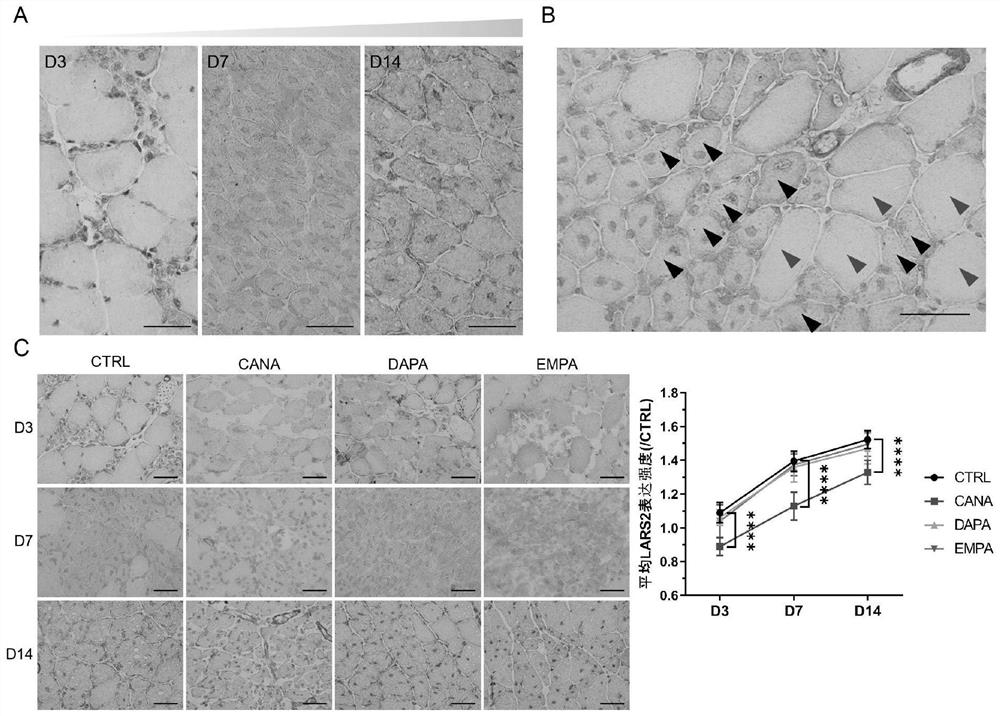
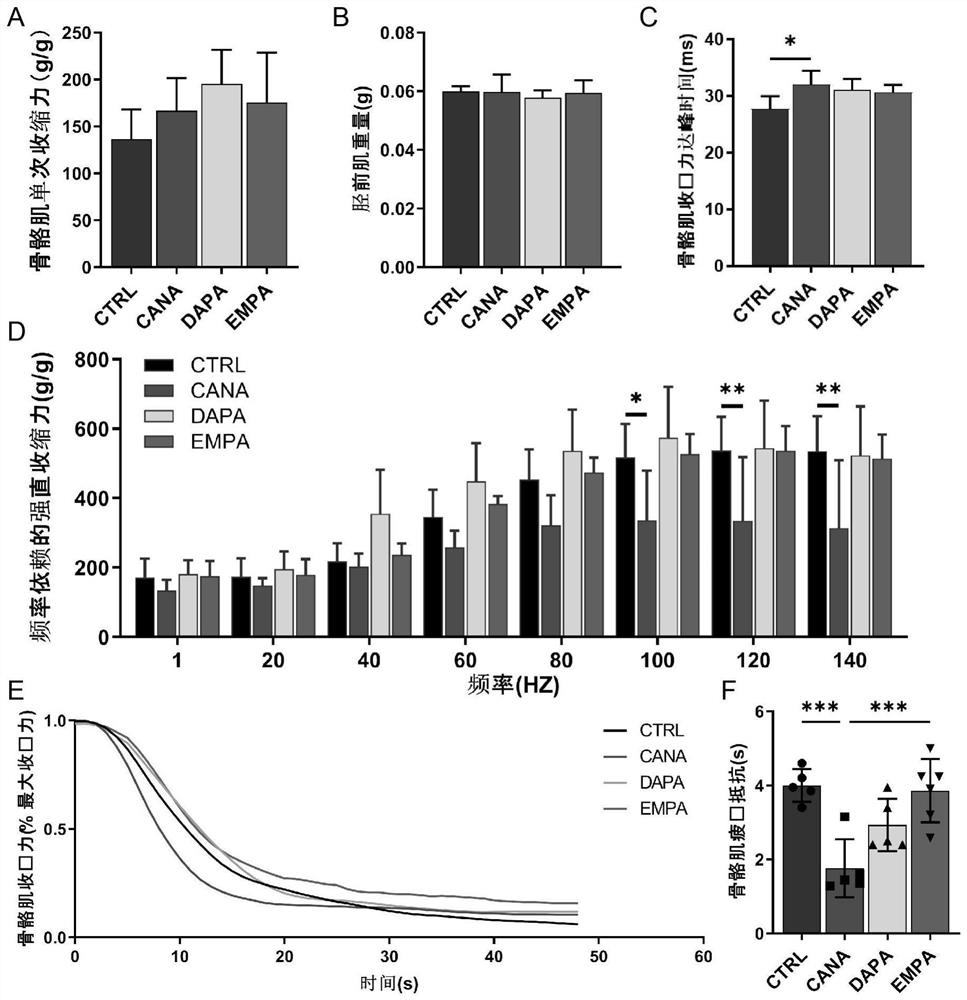
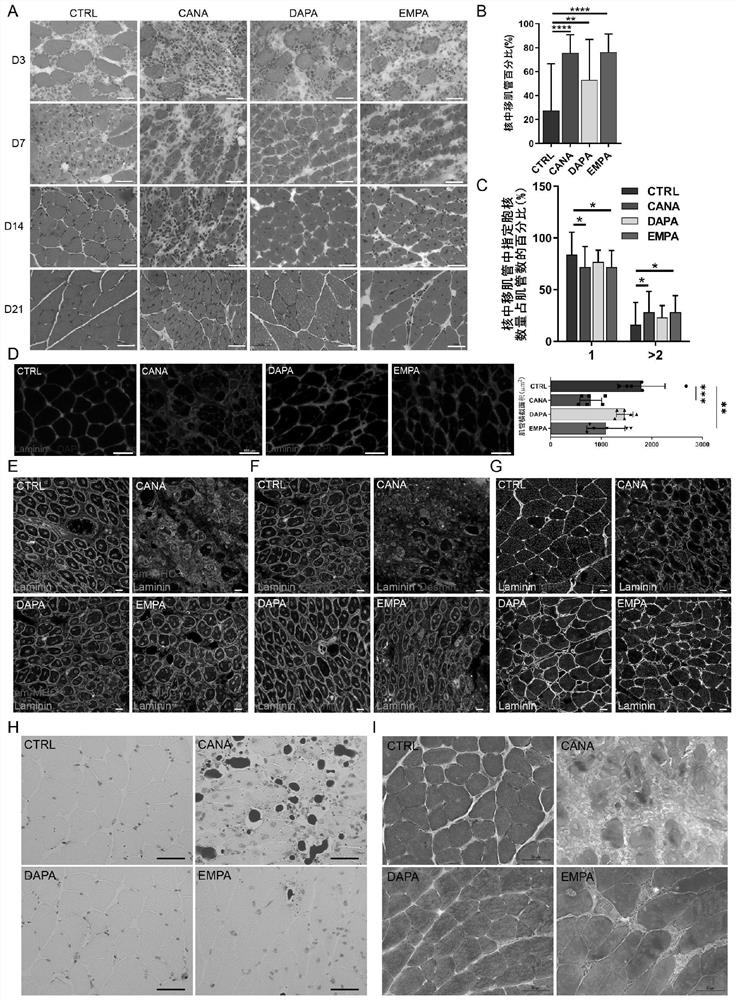
[0022] Embodiment 1 is combined with attached Figure 1-8 ; Application of leucyl-tRNA synthetase 2 to muscle repair after injury.
[0023] Application of leucyl-tRNA synthetase 2 to repair of skeletal muscle after injury.
[0024] Application of leucyl-tRNA synthetase 2 in regulating repair of damaged skeletal muscle by affecting mitochondrial function.
[0025] Application of leucyl-tRNA synthetase 2 in the repair of ischemic injured skeletal muscle.
[0026] Use of leucyl-tRNA synthetase 2 in myoblast differentiation.
[0027] Use of leucyl-tRNA synthetase 2 in the maintenance of mitochondrial stability.
[0028] Leucyl-tRNA synthetase 2 is LARS2.
Embodiment 2
[0029] Embodiment 2, in conjunction with attached Figure 1-4 , the application of leucyl-tRNA synthetase 2 in the repair of ischemic injured skeletal muscle,
[0030] To clarify the role of LARS2 in the repair of ischemic injured skeletal muscle;
[0031] 1. The study used canagliflozin, which can down-regulate LARS2 protein, or a blank control orally orally administered C57BL / 6 mice for 7 days, and then ligated the right femoral artery to create a lower limb ischemia model. Then the mice continued to gavage canagliflozin or blank control for 3 / 7 / 14 days;
[0032] 2. The mice were anesthetized 14 days after the ischemic injury and the tibialis anterior muscle was isolated to detect the functional repair of the ischemic injured skeletal muscle;
[0033] 3. The tibialis anterior muscle and gastrocnemius muscle of the mouse were collected on 3 / 7 / 14 days after the ischemic injury, and frozen sections were made. Sections were used to observe skeletal muscle repair.
Embodiment 3
[0034] Embodiment 3, in conjunction with attached Figure 1-4 , the application of leucyl-tRNA synthetase 2 in the repair of ischemic injured skeletal muscle,
[0035] 1. Six-week-old male C57BL / 6 mice were fed with a high-fat diet (60kcal%; Beijing Botai Hongda Biotechnology Co., Ltd.) for 6-8 weeks to make them obese and abnormal glucose tolerance. Then streptozotocin was used for continuous intraperitoneal injection at 40 mg / kg for 4 days (the mice were fasted for 12 hours before each injection). After the above-mentioned injections, the mice should pay attention to changing cages and keeping them clean to avoid infection. After continuing to feed for 1 week, the weight of the mice gradually recovered and a stable increase in blood sugar appeared. A blood sugar over 250 mg / dl was considered a successful model.
[0036] 2. The above-mentioned mice were divided into a control group and a canagliflozin (CANA) group (specifically inhibiting the expression of LARS2 in skeleta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com