Preparation method of cyanopropyne derivative, liquid crystal medium and liquid crystal lens
A liquid crystal lens and derivative technology, applied in the field of liquid crystals, can solve the problems of limiting the development of liquid crystal lenses and low birefringence, achieve good optical and chemical stability, increase birefringence, and low rotational viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
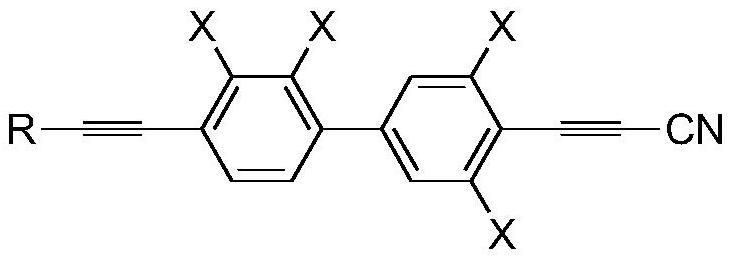
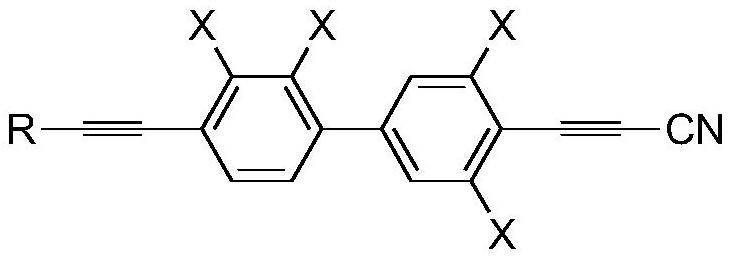
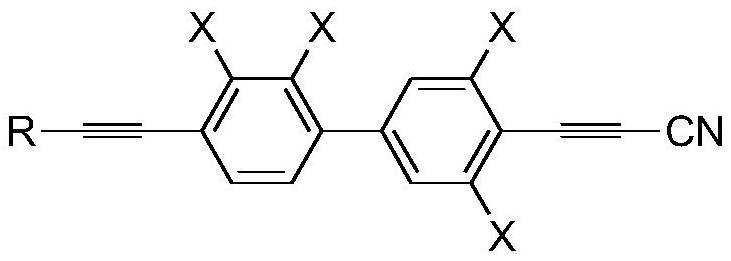
[0077] This example provides a preparation method of propyne cyanide derivatives, and prepares corresponding propyne cyanide derivatives. Specifically, the preparation method includes the following steps:
[0078] Step (1): In a four-necked round-bottomed reaction flask protected by an argon atmosphere, add tetrahydrofuran and triethylamine, start stirring, and then add the compound of general formula I, the compound of general formula II, triphenylphosphine, and oxynitride in sequence copper. Among them, the compound of general formula I is The compound of general formula II is
[0079] And do anaerobic treatment three times to the reaction system, under N 2 Under protection purge, add catalyst Pd(Ph 3 P) 2 Cl 2 , the reaction system was heated up to 40°C, and after 30 minutes of reaction, the reaction system was heated to 60°C, reacted for six hours, stopped the reaction, and cooled to room temperature. At room temperature, the reaction system was suction-filtered,...
Embodiment 2
[0111] The difference between this embodiment and embodiment 1 is that the raw materials input are different, so the products produced in each step are different. Wherein the feeding ratio of each raw material and the preparation method are the same, please refer to the relevant content of Example 1 for details. Specifically, in this embodiment, the compound of general formula I is R is C 5 h 11 -; the compound of general formula Ⅱ is X is H; The compound of general formula III obtained in the step (1) is
[0112] In step (2), the compound of general formula IV of input is where X is F, X 1 for Cl. The compound of general formula V obtained in step (2) is where X 1 The X on the attached benzene ring is F, and the remaining Xs are H.
[0113] The compound of general formula VI obtained in step (3) is The compound of general formula VII obtained in step (4) is.
[0114] The chemical formula of the propargyl cyanide derivative that final step (5) obtains is ...
Embodiment 3
[0119] The difference between this embodiment and embodiment 1 is that: the input raw materials are different, so the products produced in each step are different. Wherein the feeding ratio of each raw material and the preparation method are the same, and details can be referred to the relevant content of Example 1. Specifically, in this embodiment, the compound of general formula I is R is C 3 h 7 -; the compound of general formula Ⅱ is X is F; The compound of general formula III obtained in the step (1) is
[0120] In step (2), the compound of general formula IV of input is where X is H, X 1 for Br. The compound of general formula V obtained in the step (2) is where X 1 The X on the attached benzene ring is H, and the remaining X is F.
[0121] The compound of general formula VI obtained in step (3) is The compound of general formula VII obtained in step (4) is.
[0122] The chemical formula of the propargyl cyanide derivative that final step (5) obtains is...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap